·论 著·
冠心病合并慢性肾病患者应用重组人脑利钠肽预防造影剂加重肾脏损害的机制研究
王鸿超1,何 芳2,刘金明1*,谢亚囡1,李 芳1,王文素3
(1.河北医科大学第二医院心血管内科,河北 石家庄 050000;2.河北省威县中医院内科,河北 邢台 054700;3.河北省保定市第三中心医院肾内科,河北 保定 071000)
[摘要] 目的探讨合并中度慢性肾功能不全的不稳定型心绞痛患者在接受冠状动脉造影(coronary angiography,CAG)及非急诊经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)时,重组人脑利钠肽(recombinant human brain natriuretic peptide,rhBNP)对造影剂肾病(contrast-induced nephropathy,CIN)的预防作用。方法将209例入选患者随机分为:水化组(103例)于术前12 h至术后12 h给予0.9%氯化钠1.0 mL·kg-1·h-1静脉滴注;rhBNP组(106例)于术前24 h给予低剂量rhBNP(0.005 μg·kg-1·min-1)静脉滴注。分别于术前和术后24 h、48 h、1周 、1个月测定胱抑素C(Cystatin C,Cys C)、血清肌酐(serum creatinine,SCr)、肾小球滤过率(estimated glomerular filtration rate,eGFR)等指标。并于术前、术后24 h检测血清肿瘤坏死因子α(tumor necrosis factor,TNF-α)和醛固酮(aldosterone,ALD)。主要终点为CIN发生率,次要终点观察Cys C、SCr、 eGFR及TNF-α、ALD手术前后的变化。结果rhBNP组CIN发生率8.5%显著低于水化组的23.3%(P<0.01),无论是行CAG还是PCI(均P<0.05)。2组Cys C均于术后24 h升高达峰值,术后1个月恢复至术前水平,但rhBNP组升高幅度比水化组小。2组SCr及eGFR均于术后24h开始升高或下降:水化组SCr及eGFR于术后1周达到最大变化值,1个月恢复至术前水平;而rhBNP组于术后48 h达到最大变化值,1周恢复至术前水平,且SCr及eGFR变化幅度更小。2组术后TNF-α和ALD较术前均有明显升高(P<0.05),水化组升高更明显(P<0.05)。结论于CAG或非急诊PCI术前使用低剂量rhBNP预防CIN安全、有效,其效果优于水化治疗。rhBNP能减轻肾功能损伤程度,并且缩短肾功能恢复时间。rhBNP预防CIN的作用可能是通过抑制炎症反应和肾素-血管紧张素-醛固酮系统的机制实现的。
[关键词] 肾病;冠状血管造影术;利钠肽 doi:10.3969/j.issn.1007-3205.2016.10.009
造影剂肾病(contrast-induced nephropathy,CIN)是冠状动脉造影(coronary angiography,CAG)及经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)中一个重要的并发症,是急性肾损伤(acute kidney injury,AKI)的重要原因。CIN的发生,可以导致住院时间延长,增加肾脏及心血管事件的发生率,增加透析及病死率[1-2]。多种内源性及外源性危险因素影响着CIN的发生,包括基础的肾功能不全、低血压、心力衰竭、糖尿病、高龄、贫血、造影剂的数量和类型等[3-7],这些因素中,基础肾功能不全是最重要的独立危险因子之一。PCI术后急性肾功能不全的发病率从2%(基础肾功能正常)波动至20%~30%(术前肌酐>176 μmol/L或20 mg/L)。所以,对于那些合并慢性肾脏疾病(chronic kidney diseases,CKD)的不稳定型心绞痛患者来说,积极预防性保护肾功能是至关重要的[8-9]。重组人脑利钠肽(recombinant human brain natriuretic peptide,rhBNP)能改善CAG及PCI围手术期的肾功能,被认为是预防CIN极有前景的药物。临床上习惯应用水化疗法预防CIN的发生[10]。目前已有研究表明,术前使用低剂量rhBNP对预防CIN有效,其效果优于水化治疗;而且即使发生CIN,rhBNP也能减轻肾功能损伤程度,并且缩短肾功能恢复正常的时间[11]。在预防合并CKD的不稳定型心绞痛患者的CIN方面,rhBNP是否同样安全、有效尚未见报道。本研究探讨rhBNP对合并中度CKD的不稳定型心绞痛患者CIN的预防作用,旨在为提出预防高危患者CIN的新方法提供临床依据。
1 资料与方法
1.1 一般资料 选择2012年10月—2015年10月于河北医科大学第二医院心血管内科住院、年龄18~80岁、拟行CAG或非急诊PCI合并中度CKD的不稳定型心绞痛患者209例。随机分为:水化组103例,男性71例,女性32例,年龄52~75岁,平均(67.6±6.8)岁;rhBNP组106例,男性73例,女性33例,年龄51~76岁,平均(68.1±7.2)岁。
本研究经医院伦理委员会批准,所有入选患者均为自愿,并且签署知情同意书。
1.2 相关定义和计算公式
1.2.1 CIN CIN是指应用造影剂后48 h内出现血肌酐上升,相对值超过基础值的25%或绝对值超过基础值5 mg/L(44 μmol/L)。
1.2.2 肾小球滤过率(estimated glomerular filtration rate,eGFR)计算公式 eGFR=186.3(血清肌酐)-1.154(年龄)-0.203(女性:×0.742)。
1.2.3 中度肾功能不全分期 中度肾功能不全分期采用美国国家肾脏病基金会的《肾脏病生存质量指导》(K/DOQI)分期标准,CKD3期为中度肾功能不全:eGFR为30~59 mL·min-1·(1.73 m2)-1。
1.3 方法 水化组于CAG或非急诊PCI术前12 h至术后12 h给予0.9%氯化钠1.0 mL·kg-1·h-1静脉滴注;rhBNP组于CAG或非急诊PCI术前24 h给予低剂量rhBNP(0.005 μg·kg-1·min-1)静脉滴注。所有患者于术前及术后24 h、48 h、1周、1个月检测胱抑素C(Cystatin C,Cys C)、血清肌酐(serum creatinine,SCr)及eGFR,评估2组CIN的发生率及CAG或非急诊PCI术前后肾功能的变化情况。并观察术前、术后24 h血清肿瘤坏死因子α(tumor necrosis factor,TNF-α)和醛固酮(aldosterone,ALD)变化情况。
1.4 统计学方法 应用SPSS13.0统计软件分析数据。计量资料比较分别采用独立样本的t检验、配对t检验和重复测量的方差分析;计数资料比较采用χ2检验。P<0.05为差异有统计学意义。
2 结 果
2.1 2组CIN发病率比较 rhBNP组CIN发生率显著低于水化组,差异有统计学意义(P<0.01)。行CAG和PCI的患者中,rhBNP组CIN发生率均显著低于水化组(P<0.05)。见表1。
表1 2组术后CIN发病率比较
Table 1 Comparison of incidence of CIN between rhBNP group and hydration group B in patients undergoing CAG or PCI (例数,%)
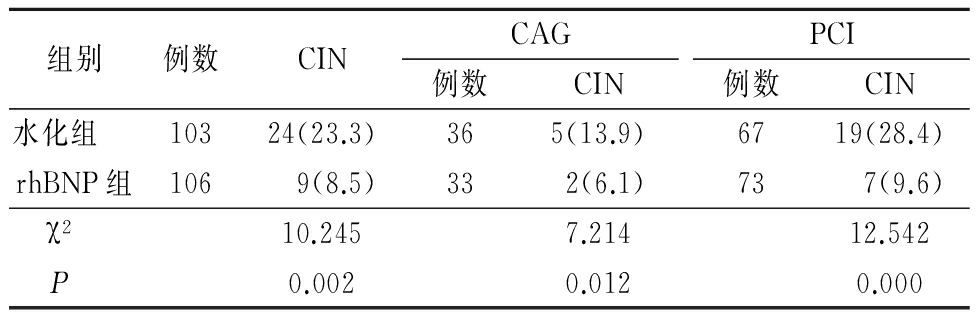
组别例数CINCAG例数CINPCI例数CIN水化组 10324(23.3)365(13.9)6719(28.4)rhBNP组1069(8.5)332(6.1)737(9.6)χ2 10.2457.21412.542P 0.0020.0120.000
2.2 2组术前、术后肾功能变化情况 2组Cys C和SCr均呈现先升高后降低的趋势,eGFR则呈先降低后升高的趋势。2组Cys C水平均于术后24 h达高峰,术后48 h逐渐回落,术后1个月恢复至术前水平,但rhBNP组升高幅度比水化组小。2组SCr水平均于术后24 h逐渐升高,水化组高峰在术后1周,术后1个月降至术前水平,rhBNP组高峰在术后48 h,术后1周降至术前水平;2组eGFR水平均于术后24 h逐渐下降,水化组谷底在术后1周,术后1个月升至术前水平,rhBNP组谷底在术后48 h,术后1周升至术前水平;rhBNP组SCr升高幅度及eGFR下降幅度更小。2组Cys C、SCr和eGFR组间、时点间、组间·时点间交互作用差异均有统计学意义(P<0.05)。见表2。
表2 2组手术前后肾功能变化比较
Table 2 Change of renal function before and after the procedure between the two groups
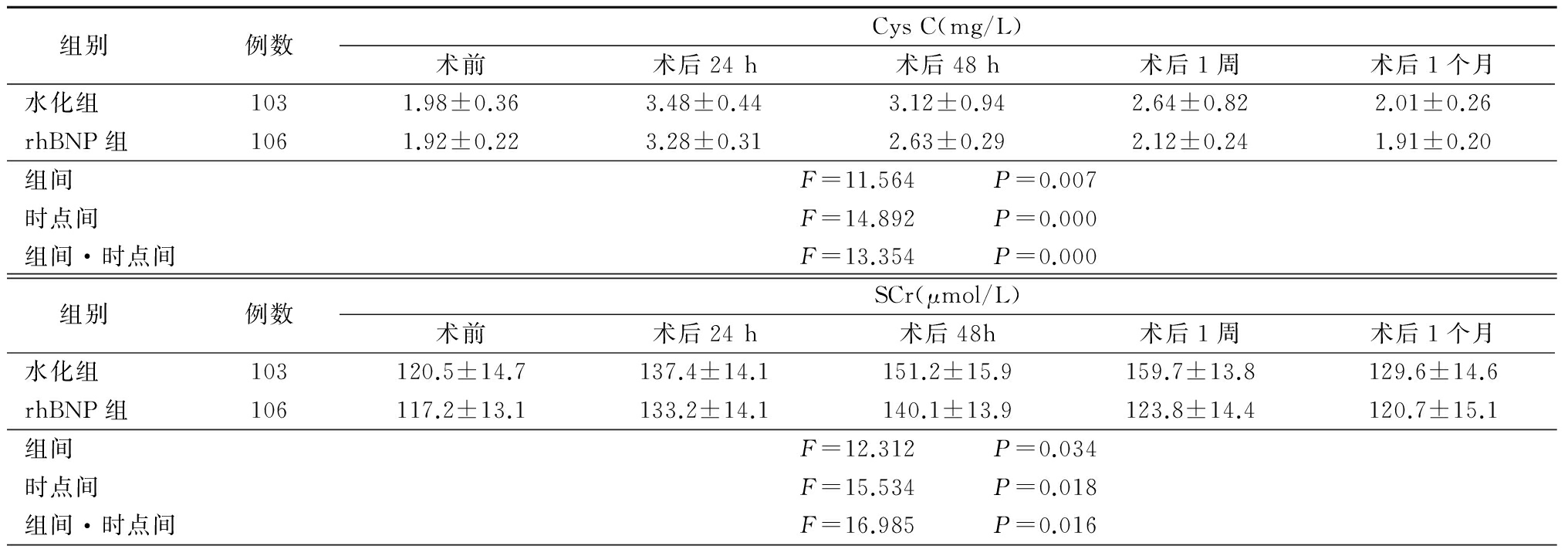
组别例数CysC(mg/L)术前术后24h术后48h术后1周术后1个月水化组1031.98±0.363.48±0.443.12±0.942.64±0.822.01±0.26rhBNP组1061.92±0.223.28±0.312.63±0.292.12±0.241.91±0.20组间F=11.564 P=0.007时点间F=14.892 P=0.000组间·时点间F=13.354 P=0.000组别例数SCr(μmol/L)术前术后24h术后48h术后1周术后1个月水化组103120.5±14.7137.4±14.1151.2±15.9159.7±13.8129.6±14.6rhBNP组106117.2±13.1133.2±14.1140.1±13.9123.8±14.4120.7±15.1组间F=12.312 P=0.034时点间F=15.534 P=0.018组间·时点间F=16.985 P=0.016
表2 (续)
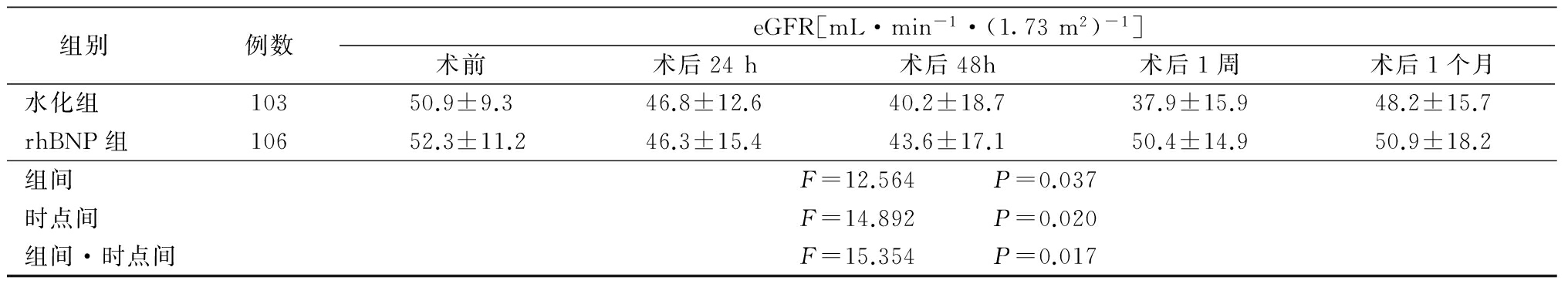
组别例数eGFR[mL·min-1·(1.73m2)-1]术前术后24h术后48h术后1周术后1个月水化组10350.9±9.346.8±12.640.2±18.737.9±15.948.2±15.7rhBNP组10652.3±11.246.3±15.443.6±17.150.4±14.950.9±18.2组间F=12.564 P=0.037时点间F=14.892 P=0.020组间·时点间F=15.354 P=0.017
2.3 2组术前、术后24 h TNF-α和ALD变化 2组术前TNF-α和ALD水平差异无统计学意义(P>0.05);2组术后24 h TNF-α和ALD均较术前明显升高(P<0.05),术后24 h水化组TNF-α和ALD 水平高于rhBNP组,差异有统计学意义(P<0.01)。见表3。
表3 2组手术前后TNF-α和ALD变化比较
Table 3 Change of TNF-α and ALD before and after CAG or PCI between the two groups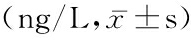
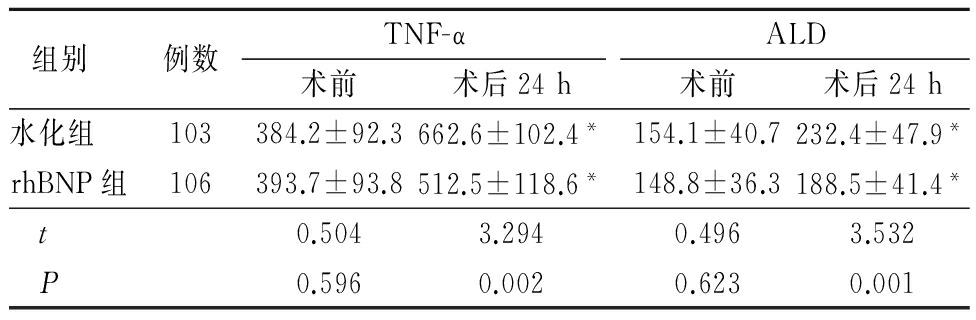
组别 例数TNF-α术前术后24hALD术前术后24h水化组103384.2±92.3662.6±102.4*154.1±40.7232.4±47.9*rhBNP组106393.7±93.8512.5±118.6*148.8±36.3188.5±41.4* t0.5043.2940.4963.532 P0.5960.0020.6230.001
*P<0.01与术前比较(配对t 检验)
3 讨 论
随着造影剂的广泛应用,CIN已成为医院获得AKI的第三大原因,仅次于肾灌注不足和肾毒性药物引起的AKI,占全部医院获得性肾衰竭的11%[12-13]。CIN的病理生理机制极其复杂,且尚不完全明确,目前研究其发生可能与以下因素有关:内源性缩血管物质如腺苷及内皮素释放增多、肾血管的收缩、造影剂的直接毒性作用、氧化应激、炎症和肾小管阻塞等;此外,造影剂产生的高渗透性可能损害肾小管细胞的运输功能及能量代谢[14-17] 。多种危险因素可以导致CIN的发生,大量研究表明CKD是患者肾功能恶化的独立危险因素[18-19]。CKD患者由于各种病理改变使得有效肾单位明显减少,肾脏的代偿能力明显减弱,轻微的血流动力学改变或肾脏损伤因素的作用,如造影剂的使用会使GFR进一步下降,进而导致发生CIN。CIN中国专家共识指出,女性SCr水平≥10 mg/L、男性SCr水平≥13 mg/L时CIN的危险增加。CKD3、4、5 期患者及eGFR<4 5 mL·min-1·(1.73 m2)-1的患者使用造影剂后CIN的风险明显增加。因此,对于基础肾功能不全的患者,预防CIN的发生,就显得尤为重要。
rhBNP已经被证实对肾功能有多种保护作用。肾脏暴露于造影剂后,会导致肾脏血流一过性增加,之后由于造影剂的直接收缩血管作用,肾素-血管紧张素-醛固酮系统、交感神经系统被激活,内源性缩血管物质腺苷、内皮素分泌增加,导致肾脏血管收缩,肾血流减少,进而损伤肾功能。而rhBNP恰恰可以抑制肾和肾素-血管紧张素-醛固酮系统、交感神经系统的激活,同时抑制内皮素释放。rhBNP还可以通过扩张入球小动脉、收缩出球小动脉,进而增加肾血流和eGFR。此外,BNP还可能减小对造影剂介导的组织缺血损伤的程度[20] 。本研究结果显示,合并中度CKD的不稳定型心绞痛患者于CAG或非急诊PCI术前预防性使用rhBNP,术后24 h及48 h,eGFR较水化组更高,而Cys C及SCr较水化组更低;CIN的发病率明显下降。
肾脏暴露于造影剂后,SCr值在24~48 h开始上升,3~5 d达峰值,7~10 d回到术前水平。然而,对于肾功能不全的患者,SCr值会出现达峰延迟,于术后7~21 d达峰[21]。Cys C是除血清SCr之外的一个新的反映eGFR变化的内源性标志物,影响因素少,其血清水平主要由eGFR决定,用其反映肾功能变化的敏感度较高[22]。本研究结果显示,2组Cys C水平均于术后24 h达峰,术后1个月恢复至术前水平,但rhBNP组较水化组的变化相对不明显;水化组SCr及eGFR均于术后1周达到最大变化值,1个月左右逐渐恢复;而rhBNP组于术后48 h达到最大变化值后即开始恢复,1周左右即逐渐恢复至术前水平,且SCr及eGFR水平的变化幅度更小。这些结果均表明预防性应用rhBNP更有利于肾功能的恢复。
另外,本研究结果显示术后rhBNP组TNF-α和ALD水平较水化组低,提示rhBNP预防CIN的作用可能是通过抑制炎症反应和肾素-血管紧张素-醛固酮系统的机制实现的,这为预防CIN提供了一种新的思路。
[参考文献]
[1] 蔡淇冰,贾国良,李三潭,等.造影剂肾病患者的远期肾功能变化与长期预后的相关性[J].岭南心血管病杂志,2015,21(5):625-627.
[2] James MT,Ghali WA,Tonelli M,et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function[J]. Kidney Int,2010,78(8):803-809.
[3] 王金艳,丁文飞,钟爱民.造影剂肾病发病相关因素研究进展[J].中国实用内科杂志,2013,33(12):984-986.
[4] Li WH,Li DY,Han F,et al. Impact of anemia on contrast-induced nephropathy(CIN) in patients undergoing percutaneous coronary interventions[J]. Int Urol Nephrol,2013,45(7):1065-1070.
[5] Schilp J,de Blok C,Langelaan M,et al. Guideline adherence for identification and hydration of high-risk hospital patients for contrast-induced nephropathy[J]. BMC Nephrol,2014,15(1):2.
[6] Jorgensen AL. Contrast-induced nephropathy:pathophysiology and preventive strategies[J]. Crit Care Nurse,2013,33(1):37-46.
[7] Liu Y,Tan N,Zhou YL,et al. The contrast medium volume to estimated glomerular filtration rate ratio as a predictor of contrast-induced nephropathy after primary percutaneous coronary intervention[J]. Int Urol Nephrol,2012,44(1):221-229.
[8] 丁琦,欧阳茂.经皮冠状动脉介入治疗患者造影剂肾病的发病率及其相关因素[J].心脏杂志,2015,27(2):142-144.
[9] Stacul F,van der Molen AJ,Reimer P,et al. Contrast induced nephropathy:updated ESUR Contrast Media Safety Committee guidelines[J]. Eur Radiol,2011,21(12):2527-2541.
[10] 王丽江,都俊华.静脉水化疗法预防合并心功能不全老年患者对比剂肾病的临床研究[J].河北医科大学学报,2013,34(3):256-258.
[11] Liu JM,Xie YN,Gao ZH,et al. Brain natriuretic peptide for prevention of contrast-induced nephropathy after percutaneous coronary intervention or coronary angiography[J]. Can J Cardiol, 2014,30(12):1607-1612.
[12] 梁柱,皮婧静,韩天瞾,等.老年急性肾损伤相关因素临床分析及探讨[J].临床荟萃,2012,27(24):2126-2129.
[13] Solomon R,Dauerman HL. Contrast-induced acute kidney injury[J]. Circulation,2010,122(23):2451-2455.
[14] 刘宇翔,郭强强,刘春.不同剂量碘海醇对大鼠肾脏氧自由基的影响[J].中国中西医结合肾病杂志,2013,14(5):396-397,后插3.
[15] 牛哲莉,李明明,杨洪娟,等.碘海醇所致造影剂肾病的研究进展[J].临床军医杂志,2015,43(8):876-878.
[16] Lingegowda V,Van QC,Shimada M, et al. Long-term outcome of patients treated with prophylactic nesiritide for the prevention of acute kidney injury following cardiovascular surgery[J]. Clin Cardiol,2010,33(4):217-221.
[17] 陈爱文,郑如义,钟思干.高敏C反应蛋白对经皮冠状动脉介入术后发生造影剂肾病相关性分析[J].广州医药,2015,46(5):61-63.
[18] Maioli M,Toso A,Leoncini M,et al. Persistent renal damage after contrast-induced acute kidney injury:incidence,evolution,risk factors,and prognosis[J]. Circulation,2012,125(3):3099-3107.
[19] Aurelio A,Durante A. Contrast-induced nephropathy in percutaneous coronary interventions:pathogenesis,risk factors,outcome,prevention and treatment[J]. Cardiology,2014,128(1):62-72.
[20] Kelesidis I,Mazurek J,Khullar P,et al. The effect of nesiritide on renal function and other clinical parameters in patients with decompensated heart failure and preserved ejection fraction[J]. Congest Heart Fail,2012,18(3):158-164.
[21] 夏豪,李宸宇,方五旺,等.急性冠脉综合征伴肾功能不全介入治疗的临床观察[J].安徽医药,2013,17(12):2070-2072.
[22] 谢华强,曹政,杨勇,等.胱抑素C对造影剂肾病早期的预测价值[J].中国老年学杂志,2015,35(24):7325-7326.
(本文编辑:许卓文)
The pathogenesis of recombinant human brain natriuretic peptide for prevention of further renal damage in patients with chronic kidney disease undergoing coronary angiography or non-emergent percutaneous coronary intervention
WANG Hong-chao1, HE Fang2, LIU Jin-ming1*, XIE Ya-nan1, LI Fang1, WANG Wen-su3
(1.Department of Cardiology, the Second Hospital of Hebei Medical University, Shijiazhuang 050000, China;2.Department of Cardiology, Traditional Chinese Medicine Hospital of Weixian,Hebei Province,Weixian 054700, China; 3.Department of Nephrology, the Third Center Hospital of Baoding City, Hebei Province, Baoding 071000, China)
[Abstract] Objective To investigate the effect of recombinant human brain natriuretic peptide (rhBNP) for prevention of contrast-induced nephropathy(CIN) in unstable angina patients with moderate chronic kidney disease(CKD) undergoing coronary angiography(CAG) or non-emergent percutaneous coronary intervention(PCI). Methods Two hundred and nine cases of selected patients were randomly divided into: Hydration group(103 cases) who received intravenous 0.9% sodium chloride with 1.0 mL·kg-1·h-1 in the preoperative and postoperative 12 h; rhBNP group(106 cases) who received the low dose rhBNP(0.005 μg · kg-1·min-1) in the preoperative 24 h.Cystatin C(Cys C), serum creatinine(SCr) and estimated glomerular filtration rate (eGFR) levels were collected before procedure, at 24 h, 48 h, 1 week and 1 month after procedure. And we detected tumor necrosis factor(TNF-α)and aldosterone(ALD) in the preoperative and postoperative 12 h . The primary outcome was CIN incidence. The secondary endpoint was the changes in the Cys C, SCr, eGFR, TNF-α and ALD before and after procedure. Results The incidence of CIN in patients on rhBNP group was 8.5%, which was significantly lower than those on hydration group of 23.3%(P<0.01), whether performing CAG or PCI(all P<0.05). Cys C of the two groups was elevated to peak at postoperative 24 h, and returned to the preoperative levels one month after operation. But rhBNP group elevated to a lesser extent than hydration group. The SCr and eGFR of the two groups began to increase or decrease at postoperative 24h, hydration group achieved to maximum change at postoperative 1 week, and returned to the preoperative levels one month after operation. rhBNP group achieved to maximum change at postoperative 48 h, and returned to the preoperative levels one week after operation. And the variation width of SCr and eGFR was smaller. After operation the levels of TNF-α and ALD were significantly higher compared with before operation(P<0.05), hydration group increased more significantly(P<0.05). Conclusion Exogenous administration of low dose of rhBNP before CAG or non-emergent PCI has a protective effect on renal function and can significantly decrease the incidence of CIN, the effect is better than hydration treatment. rhBNP can reduce the degree of renal damage and shorten the time of recovery of renal function. The effect of prevented CIN of rhBNP may be through the mechanism of inhibiting the inflammatory response and the renin-angiotensin-aldosterone system.
[Key words] nephrosis; coronary angiography; natriuretic peptide
[收稿日期] 2016-07-12;
[修回日期]2016-08-12
[作者简介] 王鸿超(1982-),男,河北石家庄人,河北医科大学
*通讯作者:。E-mail:liujinming74@163.com
[中图分类号] R256.59
[文献标志码]A
[文章编号]1007-3205(2016)10-1149-05
第二医院主治医师,医学硕士,从事心血管内科疾病诊治研究。



