表1激光诱导CNV在不同时间的发生率
Table1Theincidencerateoflaser-inducedCNVindifferenttime(例数,%)
·论著·
李 凡,尚庆丽*,郝玉华,马景学,叶存喜,王 鑫
(河北医科大学第二医院眼科,河北 石家庄 050000)
[摘要]目的补体旁路途径在脉络膜新生血管(choroidal neovascularization,CNV)的发生发展中起重要作用,补体因子B(complement factor B,CFB)作为补体旁路途径的重要因子可成为阻断补体活化的靶点。本研究探讨重组CFB-siRNA在实验性CNV中的抑制作用与机制。方法取Brown Norway大鼠45只随机分为空白对照组、实验对照组、实验组各15只(30只眼)。空白对照组不给予任何干预措施,实验对照组与实验组均激光光凝建立大鼠CNV模型。实验对照组在CFB表达高峰前日尾静脉注射生理盐水0.5 μL,实验组同一时间尾静脉注射CFB-SiRNA(0.5 μL/75 μg),均隔日注射1次,共注射3次。各组分别于光凝后3、7、14、21、28 d进行荧光素眼底血管造影(fluorescein fundusangiography,FFA),根据荧光素渗漏程度对各光凝斑评分并检测CNV的生长情况;采用免疫组织化学法检测各组视网膜脉络膜组织中CFB、血管内皮生长因子(vascular endothelial growth factor,VEGF)、碱性成纤维细胞因子(basic fibroblast growth factor,BFGF)的表达情况,并测定其灰度值。结果FFA显示:实验组与实验对照组比较,在光凝后7、14、21、28 d CNV发生率差异有统计学意义(P<0.05)。免疫组织化学检测结果显示:空白对照组正常大鼠CFB、VEGF和BFGF在视网膜和脉络膜中的表达非常弱。CFB表达情况:光凝后3 d实验组与实验对照组CFB表达达最高峰,随后表达减少,光凝后7 d仍有少量表达,光凝后14~28 d表达趋于稳定。实验对照组VEGF、BFGF在光凝后7~14 d表达明显增多,光凝后21 d达高峰;实验组VEGF、BFGF在光凝后14、21、28 d表达减少。3组CFB、VEGF和BFGF灰度值在组间、时点间、组间·时点间交互作用差异均有统计学意义(P<0.05)。结论尾静脉注射CFB-siRNA可抑制实验性CNV的发展,通过抑制CFB,阻断补体旁路途径,减少CNV形成过程中VEGF、BFGF的表达。
[关键词]脉络膜新生血管化;补体因子B;血管内皮生长因子
doi:10.3969/j.issn.1007-3205.2017.11.015
脉络膜新生血管(choroidal neovascularization,CNV)受多种因素调控[1-6]。关于CNV的确切发病机制至今尚未明确,也缺少理想有效的治疗手段。近期研究发现补体旁路途径的激活在实验性CNV的发展中起关键作用[7],旁路途径中补体因子B(complement factor B, CFB)可以作为一个重要的靶点来阻断补体活化的旁路途径。本研究采用RNAi技术,用CFB-siRNA将CFB沉默,从而阻断旁路途径,观察CFB-siRNA对CFB、血管内皮生长因子(vascular endothelial growth factor,VEGF)、碱性成纤维细胞因子(basic fibroblast growth factor,BFGF)的抑制作用,旨在为CNV的治疗开辟新的思路。
1.1 CNV动物模型的制备 健康8~10周雄性Brown Norway(BN)大鼠45只(购于北京维通利华实验动物技术有限公司),体质量180~200 g,常规喂养,实验前经裂隙灯、眼底镜检查双眼均正常。10%水合氯醛(0.35 mL/100 g体质量)腹腔注射麻醉,盐酸丙美卡因滴眼液眼表麻醉,1.5 cm×3.0 cm载玻片作为角膜接触镜,应用Coherent Novus-Omni氪离子激光治疗机(美国Coherent公司)进行激光光凝,光凝参数为:波长647 nm,光斑直径50 μm,功率260 mW,曝光时间0.05 s,距视盘2~3个视盘直径均匀光凝9~10个点。激光光凝时产生气泡者为击穿Bruch膜的标志。
1.2 方法
1.2.1 动物分组 45只大鼠随机分为3组,每组15只、30只眼,分别为空白对照组、实验对照组、实验组。空白对照组不给予任何处理,实验对照组及实验组大鼠均氪激光光凝建立CNV模型。实验对照组在CFB表达高峰前尾静脉注射生理盐水0.5 μL;实验组在CFB表达高峰前日尾静脉注射CFB-SiRNA(0.5 μL/75 μg)。注射时间:隔日注射1次,共注射3次。
1.2.2 荧光素眼底血管造影检查 于光凝后3、7、14、21、28 d,随机选取各组大鼠3只,腹腔注射10%荧光素钠注射液(0.5 mL/kg),进行荧光素眼底血管造影(fluorescein fundusangiography,FFA),观察荧光素渗漏范围及程度,将各时间点出现的CNV光凝斑数目除以该时间点的光凝斑总数(百分数),记为该时段CNV的发生率。
1.2.3 免疫组织化学法检测相关因子在视网膜脉络膜中的表达 各组在上述时间点完成FFA检查后6 h,待大鼠体内荧光素染料排净,使用过量的水合氯醛腹腔注射处死大鼠,随即摘取眼球,固定于FAA固定液中。乙醇梯度脱水,全层石蜡包埋,修整,4~5 μm连续切片。按照试剂盒说明书行CFB、VEGF、BFGF免疫组织化学检测。一抗分别为:兔多克隆抗B因子抗体,工作浓度0.5 g/L(美国Santa Cruz Biotechnology公司);兔抗鼠单克隆抗VEGF抗体,工作浓度1 g/L(北京中杉金桥生物技术有限公司);兔多克隆抗BFGF抗体,工作浓度1 g/L(北京中杉金桥生物技术有限公司)。0.01 mol/L柠檬酸盐缓冲液高温高压修复法修复抗原,采用SABC法染色,AEC显色,经苏木素复染后封片。于光学显微镜下进行100倍和400倍显微照相,采集阳性片。采用HPIAS-1000型高清晰度彩色病理图文分析系统观察染色结果,测定灰度值。
1.3 统计学方法 应用SPSS 16.0软件分析数据。计量资料比较采用重复测量的方差分析;计数资料比较采用χ2检验。P<0.05为差异有统计学意义。
2.1 FFA检查结果 光凝后3 d,实验组及实验对照组的激光斑部位早期均呈弱荧光,晚期荧光增强,荧光素渗漏不明显,CNV的发生率差异无统计学意义(P>0.05);光凝后7 d,2组激光光斑部位均有轻度荧光渗漏,实验组CNV发生率低于实验对照组,差异有统计学意义(P<0.05);光凝后14 d,2组激光光斑均呈强荧光,后期荧光素渗漏明显,CNV发生率差异有统计学意义(P<0.05);光凝后21 d,实验对照组与实验组激光光斑部位出现清晰的不规则颗粒状、环状及条状荧光(图1,2),晚期2组均形成边界略模糊的高荧光区(图3,4),形如花瓣状的2组CNV发生率均达高峰,但实验组CNV发生率显著低于实验对照组,差异有统计学意义(P<0.05);光凝后28 d,2组激光光斑仍有荧光渗漏,实验组CNV发生率仍低于实验组,差异有统计学意义(P<0.05)。见表1。
表1激光诱导CNV在不同时间的发生率
Table1Theincidencerateoflaser-inducedCNVindifferenttime(例数,%)

2.2 CFB、VEGF和BFGF免疫组织化学结果 实验对照组CFB免疫组织化学检测:光凝后CFB阳性染色信号见于光凝损伤区的视网膜全层,损伤区光斑内CFB表达不均匀,光斑近脉络膜部位CFB阳性信号的强度明显高于光斑内部(图5);光凝后3 d,光斑区可见大量红色的团状CFB阳性反应物,阳性表达达到最高峰(图6);光凝后7 d,光斑区内CFB阳性反应物减少(图7);2~4周时阳性表达趋于稳定(图8)。CFB表达高峰为光凝后3 d。3组CFB、VEGF和BFGF灰度值在组间、时点间、组间·时点间交互作用差异均有统计学意义(P<0.05)。见表2~4。
表2CFB各时间点灰度值
Table2GreylevolofCFBindifferenttime![]()
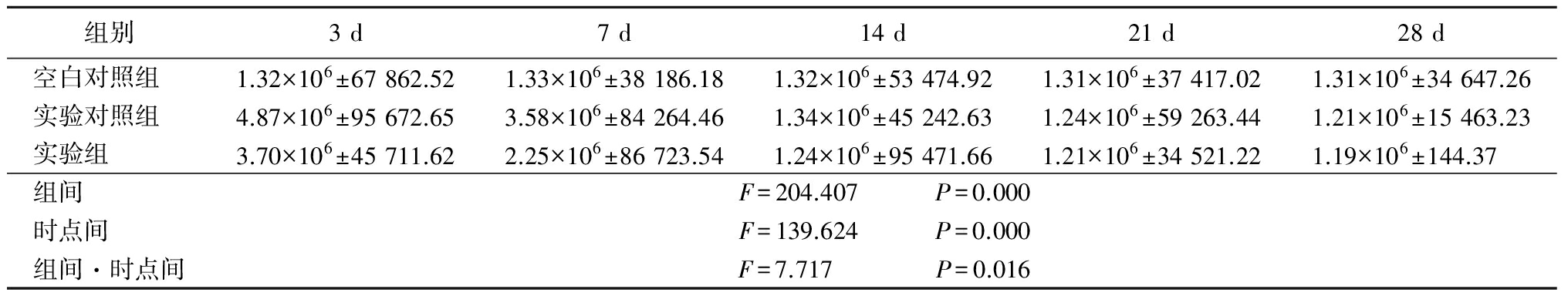
表3VEGF各时间点灰度值
Table3GreylevolofVEGFindifferenttime![]()
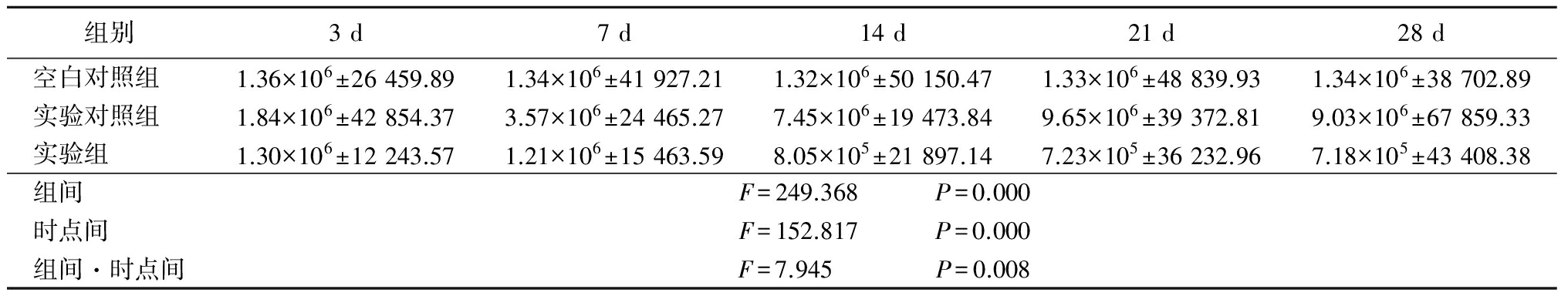
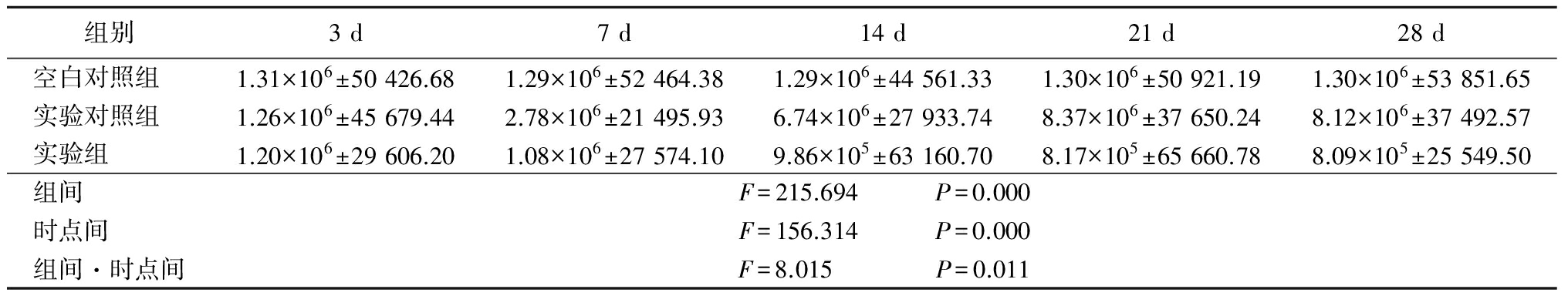
表4BFGF各时间点灰度值
Table4GreylevolofBFGFindifferenttime![]()
CNV可见于多种眼科疾病,进一步探索其发病机制及临床治疗手段一直是眼科学者的关注重点。目前应用于临床上的治疗方法主要是对于已经生成的CNV具有一定的作用,并且需要反复多次注射,并不能从根源上防止CNV的发生、发展和复发。研究表明补体旁路途径异常激活是黄斑区发生病变的诱因,可以引起黄斑萎缩、变性和新生血管形成[8-10]。CFB是补体旁路途径的重要因子,本研究采用前期研究[11]成功构建的CFB-siRNA将CFB沉默,阻断补体旁路途径,通过观察VEGF、BFGF因子的变化,探讨CFB-siRNA对实验性CNV的抑制作用,为从根本上治愈CNV提供可能性。
随着对年龄相关性黄斑变性的不断探索,研究者们发现补体旁路途径及经典途径的多个重要因子均与CNV密切相关,如CFB、CFH、C2和C3[12-15]。其中C3作为补体旁路途径激活的中心环节,是补体系统中最重要、含量最丰富的一个因子。CFB是C3激活剂前体,C3形成的C3b与CFB结合,旁路途径就会被激活,随后经一系列瀑布式反应,形成膜攻击复合物(membrane attack complex,MAC)而导致细胞裂解[16]。那么,构建CFB-siRNA将CFB沉默后,即可阻止C3b与CFB的相互作用,从而抑制旁路途径C3的形成,同时减少旁路激活途径中活化的C3转化酶中的Bb片段,使之失去酶活性,加速C3转化酶衰变。本研究空白对照组CFB在视网膜脉络膜组织中的表达非常弱,且随时间的改变CFB无明显变化;而实验对照组光凝后损伤部位出现了大量CFB表达,在光凝后3 d达高峰,随后表达逐渐减少,在光凝后7 d仍有少量表达。CFB作为补体旁路途径的重要因子,光凝后3 d实验对照组中CFB表达达最高峰,说明了实验性CNV形成过程中伴随着补体旁路途径的激活。提示可通过CFB作为靶点阻断补体旁路激活途径,从而影响CNV的形成与发展。
免疫组织化学检测结果显示,空白对照组大鼠VEGF、BFGF在视网膜脉络膜组织中的表达非常弱,且各时间点均无明显变化。实验对照组光凝后7 d,两因子开始出现表达并逐渐增多,光凝后21 d达高峰。而实验组尾静脉注射CFB-siRNA后,在光凝后21 d两因子的表达水平较实验对照组显著减少,并持续至光凝后28 d。因此,笔者推测CFB与CNV的发病机制高度相关。通过氪激光破坏Bruch膜,导致视网膜的炎症反应,从而激活了补体旁路途径,增加了CFB的表达,同时诱导了VEGF、BFGF的表达上调,脉络膜毛细血管内皮细胞必然随之分化增殖,然后穿过破裂的Bruch膜进入视网膜色素上皮层下或视网膜下腔形成CNV。也就是说,CFB在旁路途径中扮演重要角色,可以对CFB的表达情况进行调控,从而干预新生血管的形成过程,最终抑制CNV的形成发展。此外,VEGF和BFGF两者关系密切,在新生血管形成的各个环节共同作用,两者表达的增多从一定意义上促使和加速了CNV发生,推动了CNV整个复杂发病机制的发展。
近年来有研究认为补体异常激活造成MAC的生成和沉积增加,是参与CNV发生发展的重要因素之一[17-18]。补体系统的激活途径有3种,包括经典途径、旁路途径和凝集素途径。补体激活后,一系列的生物学效应随之出现。C3自然水解开启了旁路途径的变化反应,经过C3、C5到C9、CFH、CFB等多个因子共同参与反应,最终形成MAC。MAC也称为C5b-9,是补体系统3条途径激活后形成的共同末端效应产物,旁路途径被激活,形成C5转化酶,C5转化酶裂解产生C5a及C5b,C5b于细胞表面结合,与C6、C7、C8、C9形成末端补体复合物,其中结合在细胞膜上的称为MAC。也就是说,MAC的不断沉积,使生长因子释放增多,膜通透性必然随之发生改变,脉络膜血管内皮细胞发生异常增生,即导致了CNV的产生[19]。但关于CNV中MAC主要依赖于哪条补体途径生成尚不明确。本研究通过沉默CFB,对实验性CNV产生抑制作用,这就意味着旁路途径是激光诱导CNV发生发展的主要途径。补体旁路途径中CFB的缺乏,促进了C3b的裂解,进而抑制C5转化酶的生成,使得MAC的沉积减少。所以,MAC的沉积可能依赖于补体旁路途径的异常激活发生改变。
在未来的研究中,需要进一步检测CFB-siRNA在体内转染的靶向性。并且本实验仅仅从蛋白水平检测相关因子的表达,尚未从核酸水平进行定量定性检测,同时在给药剂量上存在研究空间,在今后的实验中有待进一步的研究。(本文图见封三)
[参考文献]
[1] Velez-Montoya R,Oliver SC,Olson JL,et al. Current knowledge and trends in age-related macular degeneration: today's and future treatments[J]. Retina,2013,33(8):1487-1502.
[2] Lin WJ,Kuang HY. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells[J]. Autophagy,2014,10(10):1692-1701.
[3] Maguire MG,Daniel E,Shah AR,et al. Incidence of choroidal neovascularization in the fellow eye in the comparison of age-related macular degeneration treatments trials[J]. Ophthalmology,2013,120(10):2035-2041.
[4] Arcondeguy T,Lacazette E,Millevoi S,et al. VEGF-A mRNA processing,stability and teaslation:a paradigm for intricate regulation of gene expression at the post-transcriptional level[J]. Nucleic Acids Res,2013,41(17):7997-8001.
[5] Rezaei KA,Toma HS,Cai J,et al. Reduced choroidal neovascular membrane formation in cyclooxygenase-2 null mice[J]. Invest Ophthalmol Vis Sci,2011,52(2):701-707.
[6] Michels S,Kurz-Levin M. Age-related macular degeneration(AMD)[J]. Ther Umsch,2009,66(3):189-195.
[7] Wolf-Schnurrbusch UE,Hess R,Jordi F,et al. Detection of Chlamydia and complement factors in neovascular membranes of patients with age-related macular degeneration[J]. Ocul Immunol Inflamm,2013,21(1):36-43.
[8] Rohrer B,Long Q,Coughlin B,et al. A targeted inhibitor of the complement alternative pathway reduces RPE injury and angiogenesis in models of age-related macular degeneration[J]. Adv Exp Med Biol,2010,703:137-149.
[9] Buschini E,Piras A,Nuzzi R,et al. Age related macular degeneration and drusen:neuroinflammation in the retina[J]. Prog neurobiol,2011,95(1):14-25.
[10] Zhao T,Gao J,Van J,et al. Age-related increases in amyloid beta and membrane attack complex: evidence of inflammasome activation in the rodent eye[J]. J Neuroinflammation,2015,12:121.
[11] 仝欢,尚庆丽,马景学,等.重组B因子小干扰RNA对激光诱导大鼠脉络膜新生血管的抑制作用[J].中华眼底病杂志,2010,26(1):37-41.
[12] Liu X,Zhao P,Tang S,et al. Association study of complement factor H,C2,CFB,and C3 and age-related macular degeneration in a Han Chinese population[J]. Retina,2010,30(8):1177-1184.
[13] Menghini M,Kloeckener-Gruissem B,Fleischhauer J,et al. Impact of loading phase,initial response and CFH genotype on the long-term outcome of treatment for neovascular age-related macular degeneration[J]. PLoS One,2012,7(7):e42014.
[14] Tanaka K,Nakayama T,Mori R,et al. Associations of complement factor B and complement component 2 genotypes with subtypes of polypoidal choroidal vasculopathy[J]. BMC Ophthalmol,2014,14:83.
[15] Schnabolk G,Coughlin B,Joseph K,et al. Local production of the alternative pathway component factor B is sufficient to promote laser-induced choroidal neovascularization[J]. Invest Ophthalmol Vis Sci,2015,56(3):1850-1863.
[16] Gold B,Merriam JE,Zemant J,et al. Variation in factor B(BF) and Complement component 2(C2)genes is associated with age-related macular degeneration[J]. Nat Genet,2006,38(4):458-462.
[17] Liu J, Jha P, Lyzogubov VV,et al. Relationship between complement membrane attack complex,chemokine(C-C motif) ligand 2(CCL2) and vascular endothelial growth factor in mouse model of laser-induced choroidal neovascularization[J]. J Biol Chem,2011,286(23):20991-21001.
[18] Birke K,Lipo E,Birke MT,et al. Topical application of PPADS inhibits complement activation and choroidal neovascularization in a model of age-related macular degeneration[J]. PLoS One,2013,8(10):e76766.
[19] Lipo E,Cashman SM,Kumar-Singh R. Aurintricarboxylic acid inhibits complement activation,membrane attack complex,and choroidal neovascularization in a model of macular degeneration[J]. Invest Ophthalmol Vis Sci,2013,54(10):7107-7114.
LI Fan, SHANG Qing-li*, HAO Yu-hua, MA Jing-xue, YE Cun-xi, WANG Xin
(DepartmentofOphthalmology,theSecondHospitalofHebeiMedicalUniversity,Shijiazhuang050000,China)
[Abstract]ObjectiveAlternative complement pathway plays important roles in the pathogenesis and development of choroidal neovascularization(CNV). Complement factor B(CFB), an essential factor for the alternative complement pathway, has been considered as a target to block the activity of complement. In this study, we aim to investigate the inhibitive effects and the potential mechanism of recombinant CFB-siRNA in the CNV.MethodsForty-five Brown Norway rats were randomly divided into: blank control(n=15), subject to no treatment; control group(n=15), which was subject to CNV induction by laser photocoagulation, followed by administration of normal saline(0.5 μL) via caudal vein injection one day before the expression peak of CFB, and test group, which was subject to laser photocoagulation to induce CNV, followed by administration of CFB-siRNA(0.5 μL/75 μg) via caudal vein injection one day before the expression peak of CFB. The injection of normal saline and CFB-siRNA was performed every two days for thrice. Fundus fluorescein angiography(FFA) was performed on day 3, 7, 14, 21, and 28 after laser photocoagulation. Photocoagulation score was determined according to the leakage of the fluorescein, based on which to detect the growth of CNV. Expression of CFB, VEGF, and BFGF in the retina and the choroid was measured using immunohistochemical method.ResultsFFA showed that the difference of CNV incidence between the experimental group and the experimental group was statistically significant at 7, 14, 21 and 28 days after photocoagulation(P<0.05). In the blank control, the expression of CFB, VEGF, and BFGF in the retina and choroid was comparatively lower as revealed by immunohistochemical method. For the expression of CFB, peak value was obtained 3 days after photocoagulation in the experimental and control group, followed by down-regulation of CFB. Low content of CFB was still detected 7 days after photocoagulation, while the expression was stable from day 14 to day 28. In the control group, significant up-regulation was observed in the BEGF and BFGF 7-14 days after photocoagulation, and reached the peak value on day 21 after photocoagulation. In the experimental group, down-regulation of BFGF and BFGF was noticed on day 14, 21, and 28 after photocoagulation.ConclusionAdministration of CFB-siRNA via caudal vein inhibits the progression of CNV through prohibiting CFB expression, blocking the alternative complement pathway, and down-regulating the expression of VEGF and BFGF in the pathogenesis of CNV.
[Key words]choroidal neovascularization; complement factor B; vascular endothelial growth factors
[收稿日期]2016-12-26;
[修回日期]2017-06-02
[基金项目]河北省应用基础研究计划重点基础研究项目(09966111D)
[作者简介]李凡(1982-),女,河北大名人,河北省石家庄市第一医院主治医师,医学硕士,从事眼科疾病诊治研究。
*通讯作者。E-mail:qinglishang2013@sina.cn
[中图分类号]R773.4
[文献标志码]A
[文章编号]1007-3205(2017)11-1305-06
(本文编辑:刘斯静)