Table1BMDvaluesamongthethreegroups
骆 阳1,齐 璨1,罗鹏远1,梁少博1,李舒扬2
(1.河北医科大学第三医院创伤急救中心,河北省骨科生物力学重点实验室,河北 石家庄 050051;2.内蒙古医科大学中医学院骨科教研室,内蒙古 呼和浩特 010110)
[摘要]目的探讨甲状旁腺素(parathyroid hormone,PTH)(1-34)对卵巢切除(ovariectomy,OVX)大鼠腰椎间盘退变中基质金属蛋白酶1(matrix metalloproteinase-1,MMP-1)及MMP-13表达的影响。方法3月龄雌性SD大鼠30只随机分为3组:假手术(Sham)组、卵巢切除(OVX)组及卵巢切除+ PTH(1-34)(OVX+PTH)组各10只。OVX组及OVX+PTH组行双侧卵巢切除术后12周,OVX+PTH组大鼠给予皮下注射PTH(1-34)30 μg·kg-1·d-1。连续用药12周后,处死大鼠并收集标本。对L3椎体进行骨密度(bone mineral density,BMD)检测,对L4~5椎间盘行组织学观察及免疫组织化学染色分析。结果卵巢切除术后24周,OVX组椎体BMD显著低于Sham组,OVX+PTH组椎体BMD显著高于Sham组和OVX组。OVX组MMP-1及MMP-13显著高于Sham组(P<0.05),OVX+PTH组MMP-1显著低于OVX组(P<0.05),而MMP-13与OVX组比较差异无统计学意义(P>0.05)。结论皮下注射PTH(1-34)可有效抑制卵巢切除大鼠退变椎间盘中MMP-1及MMP-13的表达。
[关键词]椎间盘退行性变;甲状旁腺素;大鼠
doi:10.3969/j.issn.1007-3205.2018.01.007
椎间盘退变是引起腰背部疼痛的主要病因之一,严重影响患者的生活质量,并给家庭及社会带来巨大的经济负担[1-2]。椎间盘退变的发病机制尚不清楚,但椎间盘退变是多种因素共同作用的结果,除正常的老化过程外,椎体生物力学性能改变、椎间盘营养供给异常以及由基质金属蛋白酶(matrix metalloproteinases,MMPs)参与的细胞外基质的降解等均参与椎间盘的退变过程,其中MMPs 的活性增高起着关键作用[3]。甲状旁腺素(parathyroid hormone,PTH)(1-34)作为骨质疏松治疗药物,可以有效提高骨质疏松患者椎体骨量,促进骨质疏松性骨折的愈合,并且可以通过丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)及蛋白激酶A(protein kinase A,PKA)信号通路刺激退变椎间盘细胞的基质合成[4-8]。本研究旨在观察PTH(1-34)对卵巢切除大鼠退变椎间盘中MMP-1及MMP-13表达的影响。现报告如下。
1.1一般资料 选取3月龄雌性Sprague-Dawley(SD) 大鼠30只,SPF级(购自北京维通利华实验动物技术有限公司),随机分为假手术(Sham)组、卵巢切除(ovariectomy,OVX)组和卵巢切除+PTH(OVX+PTH)组,每组10只。所有大鼠均分笼饲养,自由摄食水。室温(24±2) ℃,自然光照,动物饲料购自北京维通利华实验动物技术有限公司。
1.2骨质疏松大鼠模型制备 用10%水合氯醛按3 mL/kg剂量对大鼠腹腔麻醉,取侧卧位,以后方髂骨嵴上2 cm、脊柱旁l cm处为中心备皮,碘伏消毒术区,铺手术巾。以后方髂骨嵴上2 cm、脊柱旁1 cm处纵行切开,依次切开皮肤、皮下组织及筋膜组织,钝性分离肌层,暴露腹腔。于切口下方脂肪组织中暴露卵巢及输卵管(成熟卵巢为淡红色菜花状,黄豆大小,与输卵管相连,表面有不规则结节状卵泡),用镊子将卵巢及其周围的软组织提起后,丝线结扎周围组织及输卵管,切除卵巢,将切除卵巢后的组织还纳回腹腔,对手术切口进行逐层缝合,关闭腹腔。应用碘伏对切口周围进行消毒。对侧卵巢切除依照此方法进行切除。Sham组只行背部切开手术,即暴露卵巢但不切除。
1.3药物干预 卵巢切除术后12周,OVX+PTH组皮下注射PTH(1-34)(美国Sigma公司),剂量为30 μg·kg-1·d-1。连续给药12周后以过量麻醉的方法处死所有大鼠,取L3椎体进行骨密度(bone mineral density,BMD)测量。L4~5节段进行Masson染色及MMP-1、MMP-13免疫组织化学染色。
1.4检测方法
1.4.1椎体BMD检测 应用QDR Discovery双能X线吸收BMD测量仪(Hologic, Bedford, MA, USA)对椎体进行BMD检测,应用小动物扫描模式进行扫描。扫描结束后,用仪器自选工具选定所测椎体的兴趣区,读出每个标本的BMD值并记录。
1.4.2椎间盘Masson染色 石蜡切片常规脱蜡至水。Weigert铁苏木素染5~10 min,水洗。1%盐酸酒精分化,流水冲洗。丽春红酸性品红染5~10分,水洗。磷钼酸处理约5 min,苯胺蓝染液复染5 min。1%冰醋酸处理1 min,95%酒精脱水。无水酒精脱水、二甲苯透明,中性树胶封固,光镜下观察。
1.4.3免疫组织化学染色 石蜡切片常规脱蜡至水。3%过氧化氢封闭,复合酶消化,滴加抗兔MMP-1或MMP-13多克隆抗体(购自武汉博士德),4 ℃冰箱过夜。滴加生物素标记的二抗。滴加链霉素抗生物素-过氧化物酶溶液。二氨基联苯胺(diaminobenzidine,DAB)显色。苏木精复染,盐酸酒精分化,梯度酒精脱水,二甲苯透明,中性树胶封片,光镜下观察。
1.5统计学方法 应用SPSS 15.0统计学软件分析数据。计量资料比较分别采用单因素方差分析和SNK-q检验。P<0.05为差异有统计学意义。
2.1椎体BMD检测结果 卵巢切除术后24周,OVX组椎体BMD显著低于Sham组,OVX+PTH组椎体BMD显著高于Sham组和OVX组,差异有统计学意义(P<0.05),说明PTH(1-34)可有效抑制卵巢切除大鼠椎体骨量丢失。见表1。
表1各组大鼠椎体BMD检测结果
Table1BMDvaluesamongthethreegroups![]()
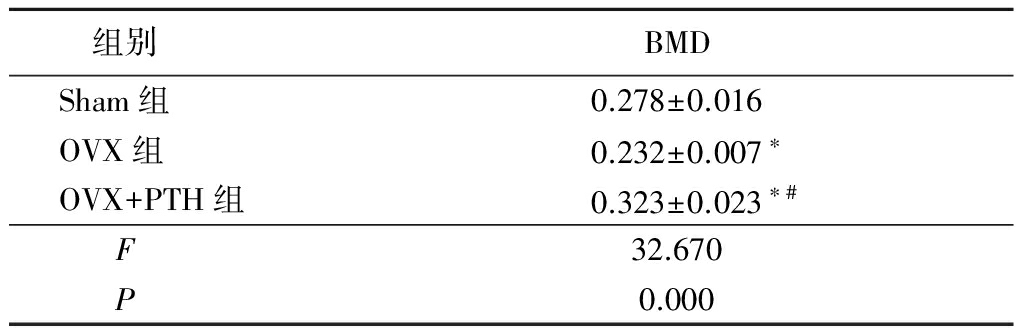
*P<0.05与Sham组比较 #P<0.05与OVX组比较(SNK-q检验)
2.2椎间盘组织学观察结果 卵巢切除术后24周,椎间盘经Masson染色结果显示,Sham组椎间盘结构正常,髓核中含有大量的脊索细胞以及细胞外基质。纤维环结构完整,胶原纤维规律排列,髓核与纤维环分界清晰。OVX组髓核中脊索细胞大量减少,由少量的、呈簇状分布的软骨样细胞所取代,髓核中可见严重的黏液样变性及泥沙样改变。纤维环结构排列紊乱,在髓核与纤维环分界区可见软骨纤维向髓核组织突出,导致髓核与纤维环组织分界不清。OVX+PTH组髓核内脊索细胞部分消失,髓核中出现少量的软骨样细胞,髓核中出现少量的黏液样变性,未见明显的泥沙样改变。在纤维环与髓核交接处可见少量纤维软骨增殖现象,髓核与纤维环组织分界较清晰。见图1。
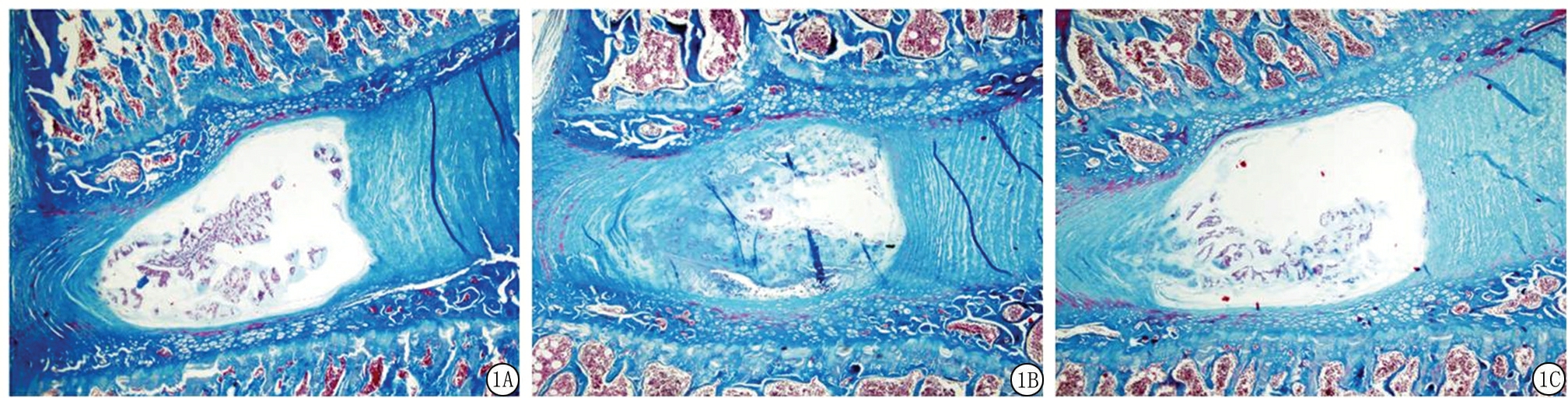
图1各组大鼠腰椎间盘Masson染色结果(×20)
A.Sham组;B.OVX组;C. OVX+PTH组
Figure1Massonstainingofintervertebraldiscsineachgroup( ×20)
2.3椎间盘免疫组织化学染色结果 MMP-1及MMP-13主要在细胞胞浆中表达。Sham组纤维环中MMP-1及MMP-13表达较弱;OVX组纤维环内MMP-1及MMP-13表达显著高于Sham组,呈强阳性表达;OVX+PTH组MMP-1表达弱于OVX组,而MMP-13表达与OVX组相比无显著变化。IOD值结果显示,OVX组MMP-1及MMP-13显著高于Sham组,OVX+PTH组MMP-1显著低于OVX组,差异有统计学意义(P<0.05);而MMP-13与OVX组比较差异无统计学意义(P>0.05)。见图2,表2。
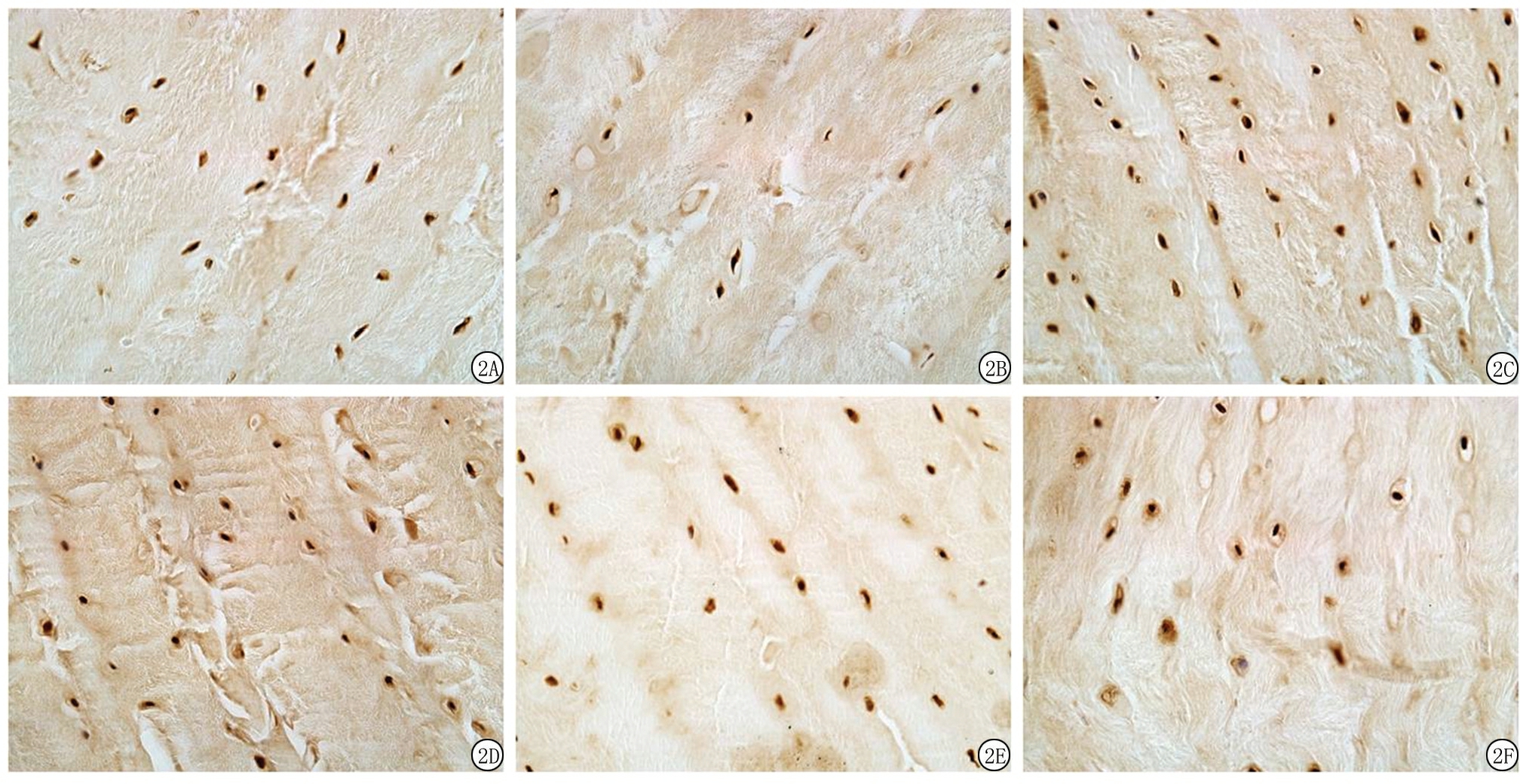
图2各组大鼠纤维环MMP-1、MMP-13免疫组织化学染色结果(×400)
A.Sham组MMP-1;B.OVX组MMP-1;C.卵巢切除+PTH组MMP-1;D.Sham组MMP-13;E.OVX组MMP-13;F.卵巢切除+PTH组MMP-13
Figure2ImmunohistochemistryassayforMMP-1andMMP-13intheannulusfibrosisamongthethreegroups( ×400)
表2各组大鼠椎间盘MMP-1、MMP-13免疫组织化学染色灰度值
Table2TheIODofMMP-1andMMP-13inimmunohistochemicalstaining![]()
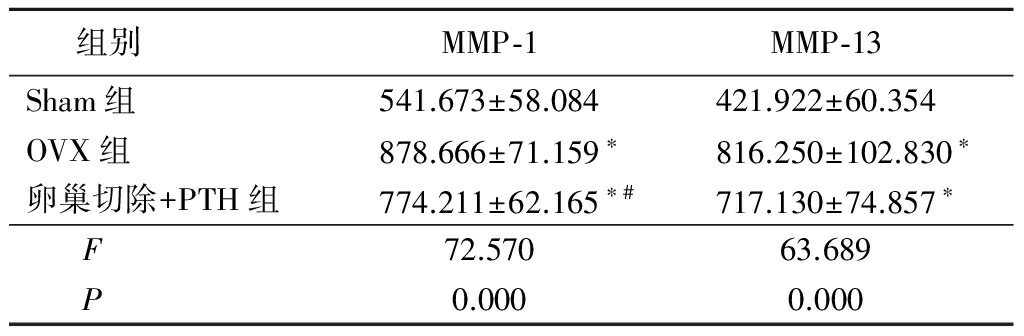
*P<0.05与Sham组比较 #P<0.05与OVX组比较(SNK-q检验)
PTH(1-34)作为促进合成代谢的药物,它不仅可以促进骨内外膜的骨形成,增加皮质骨厚度,还可刺激松质骨形成,加强松质骨与皮质骨的连接[9]。本研究结果显示,PTH(1-34)不仅可以抑制椎体骨量减少,维持脊柱结构的完整性及其生物力学特性,还可抑制椎间盘中MMP-1及MMP-13的表达,维持椎间盘细胞外基质的相对稳定性。表明PTH(1-34)可部分延缓OVX大鼠椎间盘退变进程。
椎间盘退变的具体发病机制尚不清楚,椎间盘退变的病因、发病机制成为近年来研究的重点。选择合适的动物模型是研究椎间盘退变机制的关键因素。目前,建立椎间盘退变动物模型的方法有很多种,如纤维环穿刺法[10-14]、软骨终板损伤法[15]、髓核注射消化酶法[16-19]等。卵巢切除大鼠是公认的模拟人类绝经后骨质疏松的经典动物模型。卵巢切除大鼠骨量降低与人类绝经后骨质疏松有相似之处[20]。本课题组前期研究结果显示,卵巢切除大鼠椎体骨量减少与椎间盘退变呈正相关性[21-26]。本研究结果显示,OVX组大鼠椎间盘髓核内原有的正常脊索细胞被软骨样细胞所取代,并在髓核中出现严重的黏液样变性及泥沙样改变;纤维环结构紊乱,髓核与纤维环边界不清。表明成功复制了卵巢切除大鼠椎间盘退变模型,尽管此模型不能完全模拟人类椎间盘退变过程,但在组织学改变上具有一定的相似性。
椎体结构的完整性在椎间盘退变进程中发挥着重要作用。骨质疏松可导致骨小梁数量减少、骨小梁变细甚至断裂、残存骨小梁承受负荷增加。椎间盘的营养主要通过软骨终板将椎体髓腔内血窦中的营养成分以弥散的方式进入椎间盘。Griffith等[27]应用磁共振成像技术对老年人群进行研究发现,椎体骨髓灌注量随椎体BMD降低而减少,并且对睾丸切除或卵巢切除大鼠进一步研究发现,椎体骨髓灌注量随大鼠椎体骨量的降低而出现显著性降低,椎体BMD与骨髓灌注量之间呈正相关性[28]。因此,骨质疏松可能会加速椎间盘退变。此外,有研究显示,相邻退变椎间盘之间的椎体,其骨髓灌注量较正常椎间盘之间的椎体的骨髓灌注量平均下降六分之一[29]。本研究OVX组与Sham组相比椎间盘发生明显退变的原因可能与椎体骨量显著降低、骨髓灌注量降低、椎间盘营养供给减少有关;而经PTH干预后,椎间盘退变程度减轻,可能与PTH显著增高椎体骨量、提高椎体骨髓灌注量及椎间盘营养供给有关。
尽管椎间盘退变的具体发病机制尚不清楚,但椎间盘细胞外基质代谢的变化在椎间盘退变过程中发挥着重要的作用。正常情况下,椎间盘的细胞外基质处于合成与分解代谢的动态平衡之中,该平衡的破坏可导致髓核内水分减少及其弹性的丧失,出现椎间盘退变。椎间盘含有多种胶原蛋白,包括Ⅰ型胶原、Ⅱ型胶原、Ⅲ型胶原、Ⅴ型胶原、Ⅵ型胶原蛋白等。其中Ⅰ型胶原及Ⅱ型胶原蛋白是椎间盘的重要组成成分,约占椎间盘胶原总量的80%[30]。
MMPs是降解细胞外基质的关键性蛋白水解酶,可以降解除多糖外几乎所有细胞外基质成分,在大多数生物过程中发挥着重要的作用[31-32]。MMP-1和MMP-13作为胶原酶,可以分解Ⅱ型胶原的三维螺旋结构,而且MMP-13还可以降解蛋白多糖,从而在椎间盘细胞外基质降解中发挥双重作用。正常椎间盘中MMP-1以潜伏状态存在,活化后即可解聚可溶性胶原蛋白多聚体。随着机体老化,椎间盘内环境发生改变和受到机械作用引起椎间盘细胞坏死,潜伏状态的MMP-1被激活,加速分解细胞外基质。椎间盘退变的过程中,MMPs的表达和活性均有所升高,包括MMP-1、3、7、9、13。尤其是MMP-13在退变过程中水平的升高十分明显。Salo等[33]对外伤造成的椎间盘退变动物模型进行研究发现,退变的椎间盘组织中MMP-1和MMP-2表达升高,椎体中MMP-1和MMP-2的表达也被诱导升高。由于MMP-1和MMP-2可以分解椎间盘中正常及变性的胶原,从而造成椎间盘生化特征的不稳定。Anderson等[34]研究发现手术损伤兔椎间盘6周后,椎间盘出现退变,同时椎间盘中MMP-1、MMP-8、MMP-9及MMP-13的表达水平明显升高。本研究结果显示,OVX组髓核及纤维环内MMP-1及MMP-13表达水平明显高于Sham组,经PTH(1-34)干预后,OVX+PTH组椎间盘退变程度较OVX组减轻,髓核及纤维环中MMP-1、MMP-13的表达水平明显低于OVX组。说明PTH(1-34)在一定程度上抑制椎间盘的退变进程。
综上所述,PTH(1-34)可有效抑制卵巢切除大鼠椎体BMD降低,并且可有效抑制卵巢切除大鼠椎间盘中MMP-1及MMP-13的表达。
[参考文献]
[1] 樊国峰,刘创建,王丹,等.白细胞介素-1、肿瘤坏死因子-α与腰椎间盘退变的研究进展[J].河北医科大学学报,2008,29(4):624-627.
[2] 张昭,杨学军,宋雄英,等.兔椎间失稳后弹性内固定软骨终板Ⅱ型胶原蛋白变化的实验研究[J].河北医科大学学报,2015,36(7):775-778.
[3] Le Maitre CL,Freemont AJ,Hoyland JA. Localization of degraadative enzymes and their inhibitors in the degenerate human intervertebral disc[J]. J Pathol,2004,204(1):47-54.
[4] Horwitz MJ,Augustine M,Khan L,et al.A comparison of parathyroid hormone-related protein(1-36) and parathyroid hormone(1-34) on markers of bone turnover and bone density in postmenopausal women:the PrOP study[J]. J Bone Miner Res,2013,28(11):2266-2276.
[5] Tao ZS,Zhou WS,Tu KK,et al. The effects of combined human parathyroid hormone(1-34) and simvastatin treatment on osseous integration of hydroxyapatite-coated titanium implants in the femur of ovariectomized rats[J].Injury,2015,46(11):2164-2169.
[6] Chen YJ,Wang SP,Cheng FC,et al. Intermittent parathyroid hormone improve bone microarchitecture of the mandible and femoral head in ovariectomized rats[J]. BMC Musculoskelet Disord,2017,18(1):171.
[7] Ellegaard M,Kringelbach T,Syberg S,et al. The effect of PTH(1-34) on fracture healing during different loading conditions[J]. J Bone Miner Res,2013,28(10):2145-2155.
[8] Madiraju P,Gawri R,Wang H,et al. Mechanism of arathyroid hormone-mediated suppression of calcification markers in human intervertebral disc cells[J]. Eur Cell Mater,2013,25:268-283.
[9] Zhang L,Takahashi HE,Inoue J,et a1. Effects of intermittent administration of low dose human PTH(1-34) on cancellous and cortical bone of lumbar vertebral bodies in adult beagles[J]. Bone,1997,21(6):501-506.
[10] Lei T,Zhang Y,Zhou Q,et al. A novel approach for the annulus needle puncture model of intervertebral disc degeneration in rabbits[J]. Am J Transl Res,2017,9(3):900-909.
[11] Yang CH,Chiang YF,Chen CH,et al. The effect of annular repair on the failure strength of the porcine lumbar disc after needle puncture and punch injury[J]. Eur Spine J,2016,25(3):906-912.
[12] Xin L,Zhang C,Zhong F,et al.Minimal invasive annulotomy for induction of disc degeneration and implantation of poly lactic-co-glycolic acid)(PLGA) plugs for annular repair in a rabbit model[J]. Eur J Med Res,2016,21:7.
[13] Xin L,Xu W,Yu L,et al.Effects of annulus defects and implantation of poly(lactic-co-glycolic acid)(PLGA)/fibrin gel scaffolds on nerves ingrowth in a rabbit model of annular injury disc degeneration[J]. J Orthop Surg Res,2017,12(1):73.
[14] 王字兴,蔚芃.建立纤维环穿刺法椎间盘退变模型[J].中国组织工程研究,2017,21(12):1855-1860.
[15] Fern ndez-Susavila H,Pardo-Seco JP,Iglesias-Rey R,et al. model of disc degeneration in rat tail induced through a vascular isolation of vertebral endplates[J]. J Invest Surg,2017,25:1-10.
ndez-Susavila H,Pardo-Seco JP,Iglesias-Rey R,et al. model of disc degeneration in rat tail induced through a vascular isolation of vertebral endplates[J]. J Invest Surg,2017,25:1-10.
[16] Oehme D,Ghosh P,Goldschlager T,et al. Reconstitution of degenerated ovine lumbar discs by STRO-3-positive allogeneic mesenchymal precursor cells combined with pentosan polysulfate[J]. J Neurosurg Spine,2016,24(5):715-726.
[17] Peeters M,Detiger SE,Karfeld-Sulzer LS,et al. BMP-2 and BMP-2/7 heterodimers conjugated to a fibrin/hyaluronic acid hydrogel in a large animal model of mild intervertebral disc degeneration[J]. Biores Open Access,2015,4(1):398-406.
[18] Detiger SE,Helder MN,Smit TH,et al. Adverse effects of stromal vascular fraction during regenerative treatment of the intervertebral disc:observations in a goat model[J]. Eur Spine J,2015,24(9):1992-2000.
[19] Gullbrand SE,Malhotra NR,Schaer TP,et al. A large animal model that recapitulates the spectrum of human intervertebral disc degeneration[J]. Osteoarthritis Cartilage,2017,25(1):146-156.
[20] Wronski TJ,Dann LM,Horner SL. Time course of vertebral osteopenia in ovariectomized rats[J]. Bone,1989,10(4):295-301.
[21] Tian FM,Li SY,Yang K,et al. Orally administered simvastatin partially preserves lumbar vertebral bone mass but not integrity of intervertebral discs in ovariectomized rats[J]. Exp Ther Med,2017,13(3):877-884.
[22] Liu CC,Tian FM,Zhou Z,et al. Protective effect of calcitonin on lumbar fusion-induced adjacent-segment disc degeneration in ovariectomized rat[J]. BMC Musculoskelet Disord,2015,16:342.
[23] Luo Y,Zhang L,Wang WY,et al. The inhibitory effect of salmon calcitonin on intervertebral disc degeneration in an ovariectomized rat model[J]. Eur Spine J,2015,24(8):1691-1701.
[24] Zhou Z,Tian FM,Wang P,et al. Alendronate prevents intervertebral disc degeneration adjacent to a lumbar fusion in ovariectomized rats[J]. Spine(Phila Pa 1976),2015,40(20):E1073-1083.
[25] Tian FM,Yang K,Wang WY,et al. Calcitonin suppresses intervertebral disk degeneration and preserves lumbar vertebral bone mineral density and bone strength in ovariectomized rats[J]. Osteoporos Int,2015,26(12):2853-2861.
[26] Song H,Luo Y,Wang W,et al. Effects of alendronate on lumbar intervertebral disc degeneration with bone loss in ovariectomized rats[J]. Spine J,2017,17(7):995-1003.
[27] Griffith JF,Yeung DK,Tsang PH,et al. Compromised bone marrow perfusion in osteoporosis[J]. J Bone Miner Res,2008,23(7):1068-1075.
[28] Griffith JF,Wang YX,Zhou H,et al. Reduced bone perfusion in osteoporosis:likely causes in an ovariectomy rat model[J]. Radiology,2010,254(3):739-746.
[29] Liu YJ,Huang GS,Juan CJ,et al. Intervertebral disk degeneration related to reduced vertebral marrow perfusion at dynamic contrast-enhanced MRI[J]. AJR Am J Roentgenol,2009,192(4):974-997.
[30] Roberts S,Caterson B,Menage J,et al. Matrix metalloproteinases and aggrecanase:their role in disorders of the human intervertebral disc[J]. Spine(Phila Pa 1976),2000,25(23):3005-3013.
[31] Y
 ez RJ,Porter AC.Differential effects of Rad52p overexpression on gene targeting and extrachromosomal homologous recombination in a human cell line[J]. Nucleic Acids Res,2002,30(3):740-748.
ez RJ,Porter AC.Differential effects of Rad52p overexpression on gene targeting and extrachromosomal homologous recombination in a human cell line[J]. Nucleic Acids Res,2002,30(3):740-748.
[32] Le Maitre CL,Freemont AJ,Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc[J]. J Pathol,2004,204(1):47-54.
[33] Salo J,Mackiewicz Z,Indahl A,et al. Plasmin-matrix metalloproteinase cascades in spinal response to an experimental disc lesion in pig[J]. Spine(Phila Pa 1976),2008,33(8):839-844.
[34] Anderson DG,Izzo MW,Hall DJ,et al. Comparative gene expression profiling of normal and degenerative discs:analysis of a rabbit annular laceration model[J]. Spine(Phila Pa 1976),2002,27(12):1291-1296.
LUO Yang1, QI Can1, LUO Peng-yuan1, LIANG Shao-bo1, LI Shu-yang2
(1.DepartmentofOrthopaedicSurgery,theThirdHospitalofHebeiMedicalUniversity,KeyLaboratoryofOrthopaedicBiomechanicsofHebeiProvinc,Shijiazhuang050051,China;2.DepartmentofOrthopaedicsandTraumatology,CollegeofTraditionalChineseMedicine,InnerMogoliaMedicalUniversity,Huhhot010110,China)
[Abstract]ObjectiveTo investigate the effects of parathyroid hormone(PTH)(1-34) on the expressions of matrix metalloproteinase-1(MMP-1) and MMP-13 in the intervertebral disc on an ovariectomized(OVX) rat model.MethodsThirty female Sprague-Dawley rats, 3 month of age, were randomly divided into three groups: the sham-operated(Sham) group and two ovariectomized groups treated with vehicle or PTH(1-34)(30 μg·kg-1·d-1) for twelve weeks. Treatment was started after twelve weeks post-OVX and continued for twelve weeks. At the end of experiment, bone mineral density(BMD), histology and immunohistochemistry analysis were performed to all groups.ResultsTwenty four weeks after OVX, the BMD values of L3 vertebral bodies in the OVX group were significantly decreased, compared with the Sham group(P<0.05). The BMD values in the OVX+PTH group were significantly increased, compared with the Sham group and OVX group(P<0.05). Immunohistochemistry analysis revealed a significant increase in matrix metalloproteinase(MMP)-1 and MMP-13 expression, compared with the Sham group(P<0.05), the expression of MMP-1 was significantly decreased(P<0.05), and no significant difference in MMP-13 expression in the OVX+PTH group(P>0.05), compared with the OVX group.ConclusionPTH(1-34) treatment was effective in reducing the expression of MMP-1 and MMP-13 in the degenerated disc in OVX rats.
[Key words]intervertebral disc degeneration; parathyroid hormone; rats
[收稿日期]2017-08-18;
[修回日期]2017-09-19
[基金项目]河北省医学科学研究重点课题(20170137)
[作者简介]骆阳(1982-),男,河北石家庄人,河北医科大学第三医院主治医师,医学博士,从事骨外科疾病诊治研究。
[中图分类号]R681.5
[文献标志码]A
[文章编号]1007-3205(2018)01-0029-06
(本文编辑:赵丽洁)