动脉粥样硬化(atherosclerosis,AS)的发病机制极其复杂,研究证实高同型半胱氨酸(hyperhomocysteinemia,HHcy)是AS的独立危险因素之一,HHcy参与炎症过程、过氧化、血管平滑肌增殖,促进AS形成[1-2]。口服叶酸、B族维生素是HHcy的常规治疗手段,但效果并不令人满意[3-4]。瑞舒伐他汀是目前研发的最强降脂药物,现已广泛应用于治疗AS的血脂紊乱,同时还具有稳定斑块、抗炎、抗氧化等非调制作用[5],但关于基因水平的研究仍较少,且其量效关系尚缺乏足够的循证学依据。本研究通过建立HHcy小鼠模型,检测超敏C反应蛋白(high-sensitivity C-reactive protein,hsCRP)、肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)及基质金属蛋白酶9(matrix metalloproteinases-9,MMP-9)等炎性介质及主动脉AS组织相关基因的表达,分析叶酸及不同剂量瑞舒伐他汀的干预效果,旨在为临床治疗HHcy所致AS提供新思路及理论依据。
1 材 料 与 方 法
1.1动物模型及实验分组 选择<3周龄小鼠(品系BALB/C)小鼠46只,雌雄不限,体质量10~15 g,饲养条件为清洁级,室温保持在22~24 ℃,相对湿度50%,光照时间7:00~19:00。将46只小鼠随机分为5组:对照组6只、高蛋氨酸组10只、叶酸组10只、瑞舒伐他汀低剂量组10只和高剂量组10只。对照组给予普通饲料喂养;高蛋氨酸组给予普通饲料喂养+质量分数2%的蛋氨酸;叶酸组给予高蛋氨酸饲料喂养4周后,叶酸按每天0.8 mg/kg剂量灌胃,同时持续高蛋氨酸饮食;瑞舒伐他汀低剂量组、高剂量组给予高蛋氨酸饲料喂养4周后,瑞舒伐他汀分别按每日0.8 mg/kg、1.6 mg/kg剂量灌胃,同时持续高蛋氨酸饮食。
实验动物所有操作过程均严格按照医院动物伦理委员会规程执行。本研究设计经医院伦理学委员会审查批准。
1.2观察指标
1.2.1血清Hcy与血脂、炎性指标检测 12周末,小鼠经眼静脉采血处死,留存各实验小鼠的主动脉进行指标测定。采集小鼠血液,直接静置后分离血清,-20 ℃保存,以备后续测定。留取小鼠主动脉,制作石蜡切片,并于HE染色后用图像分析测定主动脉斑块的相对面积。应用罗氏Modular P生化分析仪检测血清三酰甘油(triglyceride,TG)、总胆固醇(total cholesterol,TC)、高密度脂蛋白胆固醇(high-density lipoprotein-C,HDL-C)、低密度脂蛋白胆固醇(low-density lipoprotein-C,LDL-C)水平,试剂为罗氏配套血脂专用试剂。采用免疫荧光法检测高敏C反应蛋白(hypersensitive C-reactive protein,hsCRP),检测仪器为韩国i-CHRO-MATMREADER。采用酶联免疫吸附测定法检测Hcy、TNF-α及MMP-9,试剂盒购自美国PeproTech(派普泰克)公司。
1.2.2RNA抽提及荧光定量检测 将小鼠主动脉新鲜组织匀浆,应用Trizol试剂盒按其说明书操作步骤进行总RNA的抽提。逆转录试剂盒购自大连宝生物PrimeScriptTMRT reagent Kit(Perfect Real Time),荧光定量PCR试剂盒购自大连宝生物SYBR®Premix Ex TaqTM(Tli RNaseH Plus)。逆转录反应体系20 μL,反应条件为37 ℃ 15 min(反转录反应)、85 ℃ 5s(反转录酶的失活反应);荧光定量采用两步法,反应体系为20 μL,反应条件为预变性95 ℃,30 s。PCR反应40个循环,95℃ 3 s,60 ℃ 30 s。AS相关基因引物序列,见表1。
表1 AS相关基因引物序列
Table1Related genomic sequence of AS
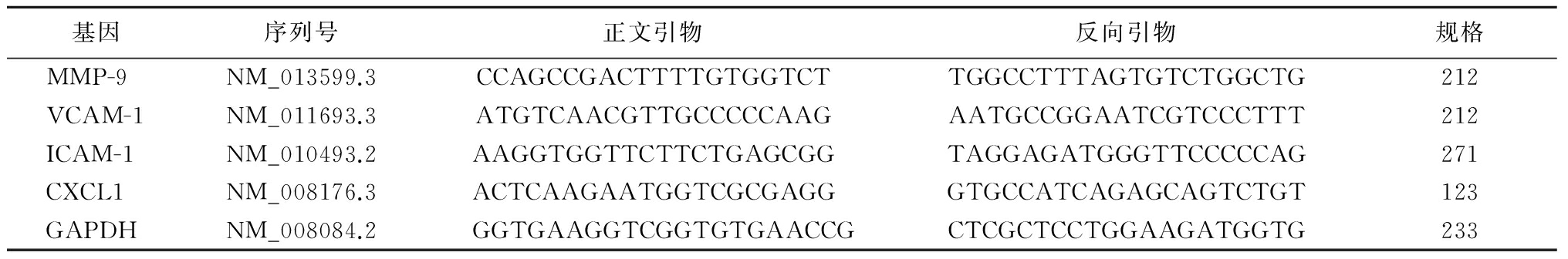
注:血管细胞黏附分子1(vascular cell adhesion molecule-1,VCAM-1),细胞间黏附分子1(intercellular adhesion molecule 1,ICAM-1),趋化因子配体1[chemokine(C-X-C motif) ligand 1,CXCL1],磷酸甘油醛脱氢酶(reduced glyceraldehyde-phosphate dehydrogenase,GAPDH)
1.3统计学方法 应用SPSS 17.0统计软件处理数据,计量资料比较分别采用单因素方差分析和SNK-q检验。P<0.05为差异有统计学意义。
2 结 果
2.1各组Hcy及血脂水平变化 高蛋氨酸组、叶酸组、低剂量组HHcy均高于对照组,叶酸组、低剂量组和高剂量组均低于高蛋氨酸组,高剂量组又低于叶酸组(P<0.05); 高蛋氨酸组、叶酸组、低剂量组TC均高于对照组,低剂量组和高剂量组低于高蛋氨酸组,高剂量组低于叶酸组和低剂量组(P<0.05);高蛋氨酸组TG高于对照组,高剂量组低于高蛋氨酸组(P<0.05); 高蛋氨酸组、叶酸组、低剂量组LDL-C高于对照组,叶酸组、低剂量组和高剂量组低于高蛋氨酸组,高剂量组低于叶酸组和低剂量组(P<0.05);高蛋氨酸组、叶酸组、低剂量组HDL-C低于对照组,高剂量组高于高蛋氨酸组、叶酸组和低剂量组(P<0.05)。见表2。
Table2Change of levels of serum Hcy and lipid among each group
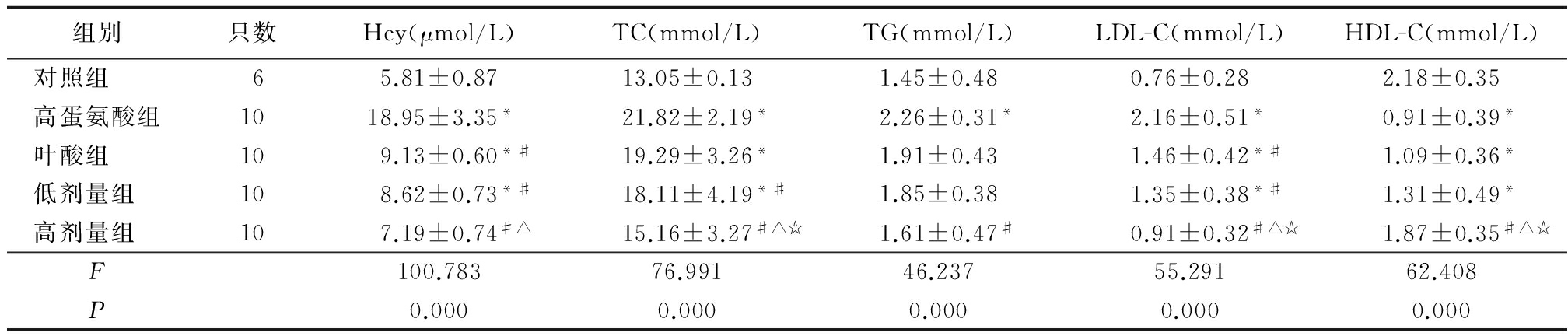
*P<0.05与对照组比较 #P<0.05与高蛋氨酸组比较 △P<0.05与叶酸组比较 ☆P<0.05与低剂量组比较(SNK-q检验)
2.2各组炎性指标比较 干预后12周,与对照组比较,高蛋氨酸组、叶酸组、瑞舒伐他汀低剂量组和高剂量组血清hsCRP、TNF-α水平明显升高,MMP-9明显下降,差异均有统计学意义(P<0.05);而高蛋氨酸组、叶酸组、瑞舒伐他汀低剂量组各炎性指标差异均无统计学意义(P>0.05);高剂量组各炎性指标明显低于高蛋氨酸组、叶酸组及低剂量组,差异均有统计学意义(P<0.05)。见表3。
Table3Comparison of inflammatory indicators among each group
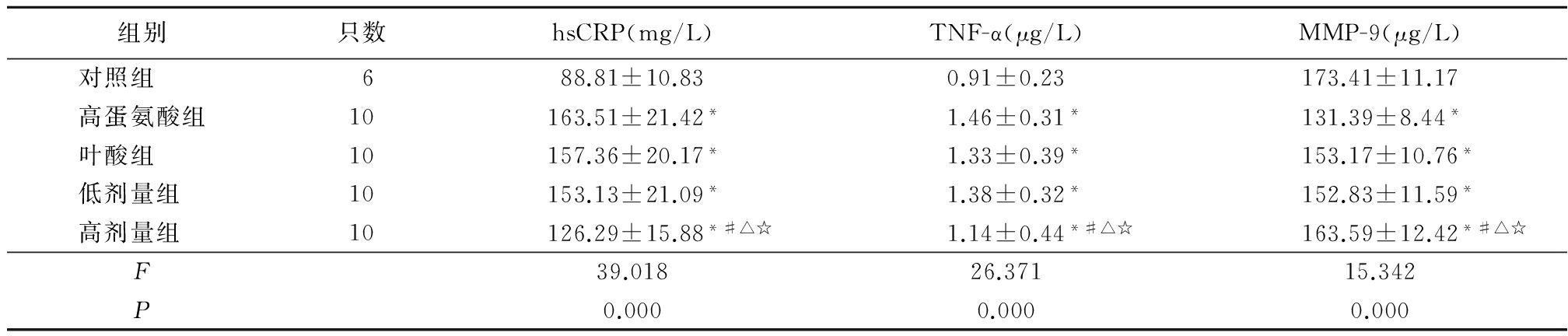
*P<0.05与对照组比较 #P<0.05与高蛋氨酸组比较 △P<0.05与叶酸组比较 ☆P<0.05与低剂量组比较(SNK-q检验)
2.3各组AS相关基因阳性细胞百分比比较 高蛋氨酸组、叶酸组、瑞舒伐他汀低剂量组和高剂量组主动脉组织CXCL1、ICAM-1、VCAM-1、MMP-9阳性细胞百分比明显高于对照组,差异均有统计学意义(P<0.05);高剂量组CXCL1、ICAM-1、VCAM-1、MMP-9阳性细胞百分比明显低于高蛋氨酸组、叶酸组和低剂量组,差异均有统计学意义(P<0.05);各AS相关基因阳性细胞百分比在叶酸组、低剂量组之间差异均无统计学意义(P>0.05)。见表4。
Table4Comparison of percentage of positive cellslevels of AS related gene among each group
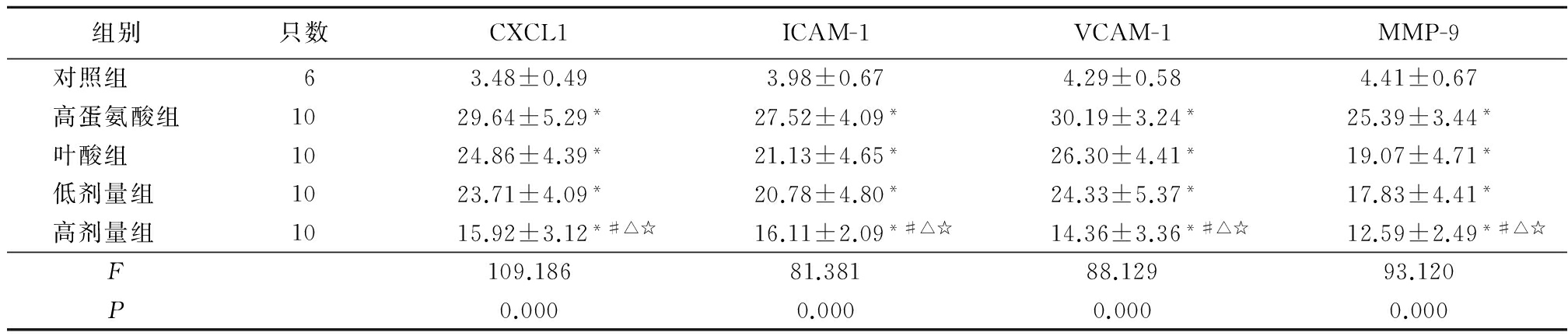
*P<0.05与对照组比较 #P<0.05与高蛋氨酸组比较 △P<0.05与叶酸组比较 ☆P<0.05与低剂量组比较(SNK-q检验)
3 讨 论
近年来,随着AS研究的深入,学者对其发病机制的观点已从以往的单纯脂质代谢紊乱所致全身动脉内壁脂质堆积逐渐转变为进展性炎症性疾病,在AS发生发展过程中,从脂质条纹演变为纤维粥样斑块,直至不稳定斑块的形成、破裂及血栓形成,均有大量炎症介质参与[6-7]。目前,临床上对HHcy治疗主要采用叶酸、B族维生素等非特异性治疗加快Hcy代谢,但往往难以逆转已经形成的AS。文献也表明,叶酸虽可降低血清 Hcy水平,但对延缓AS进展及相关并发症并无显著效果[8-9]。瑞舒伐他汀具有高效的降脂作用,不仅能防治因蛋氨酸摄入过量引发的Hcy升高,还具有内皮保护与抗炎功能,但是其具体机制不明。蒋小晶等[10]研究显示,瑞舒伐他汀可改善氧化应激水平,提高抗AS的能力,甚至逆转AS,随着瑞舒伐他汀剂量的增加,其降脂程度随之加强,对于炎症因子的抑制作用也相应加强。但何种剂量效果更佳迄今为止尚无统一认识。
本研究通过构建HHcy小鼠模型,设置不同剂量瑞舒伐他汀,并与叶酸治疗比较,结果显示干预后12周,叶酸组、瑞舒伐他汀低剂量组和高剂量组血清Hcy水平明显降低,且血脂明显改善(P<0.05);随着剂量的增加,血清Hcy水平及血脂指标进一步改善,表现出一定的剂量反应关系,说明合理范围内增加瑞舒伐他汀剂量可有效防治因蛋氨酸过度摄入所致Hcy水平升高,且其效果明显优于叶酸。考虑可能与大剂量他汀类药物加速促进Hcy代谢有关,但具体机制仍不十分明确[11]。本研究还显示,高剂量组血清hsCRP、TNF-α水平明显升高,MMP-9等炎性因子水平明显下降(P<0.05)。说明瑞舒伐他汀的剂量不仅影响降脂效果,还与抑制炎症反应密切相关,故积极强化的他汀类药物对防治AS等心血管疾病可能更有益处。
研究表明,瑞舒伐他汀可降低血清Hcy浓度及主动脉组织中Bcl-2、BAX基因表达,从而延缓HHcy大鼠主动脉AS的进程和程度[12]。CXCL1、ICAM-1、VCAM-1、MMP-9等均为AS相关基因,目前尚鲜见不同剂量瑞舒伐他汀对相关基因表达的研究。梁宇等[13]研究发现,Hcy可通过激活MAPK-ERK信号通路上调MMP-9表达,MAPK信号通路与细胞增殖和迁移过程有关,而瑞舒伐他汀可能与该信号通路活性的下调有关。本研究对相关基因的进一步分析发现,叶酸组、瑞舒伐他汀低剂量组和高剂量组主动脉组织CXCL1、ICAM-1、VCAM-1、MMP-9阳性细胞百分比明显低于高蛋氨酸组(P<0.05),且高剂量组的降幅最大,但仍高于对照组(P<0.05)。一方面表明HHcy可上调AS相关基因的表达,另一方面说明叶酸及瑞舒伐他汀可以下调该基因表达,高剂量瑞舒伐他汀效果更佳,考虑可能与抑制AKT磷酸化下调MMP-9的表达有关[14-15]。
综上所述,蛋氨酸过量摄入可诱发小鼠HHcy水平,而瑞舒伐他汀对HHcy的治疗效果具有剂量依赖性,适当提高剂量更有助于降低HHcy小鼠血清Hcy水平,改善血脂紊乱,下调AS相关基因的表达。
[参考文献]
[1] Varol E. Association between serum homocysteine and arteial stiffness:role of antihypertensive drugs[J]. J Geriatr Cardiol,2014,11(2):175-176.
[2] Loscalzo J,Handy DE. Epigenetic modifications:basic mechanisms and role in cardiovascular disease(2013 Grover Conference series)[J]. Pulm Circ,2014,4(2):169-174.
[3] 杨博,李平,孟立平,等.瑞舒伐他汀抑制同型半胱氨酸诱导的大鼠主动脉血管平滑肌细胞表型转化及信号通路[J].西安交通大学学报:医学版,2016,37(4):506-512.
[4] 吕文轩,李文耀,范思佳,等.瑞舒伐他汀对载脂蛋白E基因敲除小鼠动脉粥样硬化的治疗作用及其机制[J].吉林大学学报:医学版,2016,42(2):240-244.
[5] 余欣,路航,张瑞丽,等.瑞舒伐他汀防治动脉粥样硬化的机制研究进展[J].山东医药,2014,12(31):88-90.
[6] Guo HY,Xu FK,Lv HT,et al. Hyperhomocysteinemia independently causes and promotes atherosclerosis in LDL receptor-deficient mice[J]. J Geriatr Cardiol,2014,11(1):74-78.
[7] 刘敏,闫杰,张苏川,等.强化瑞舒伐他汀对冠状动脉造影检查时造影剂肾病的预防作用[J].川北医学院学报,2015,30(3):377-380.
[8] Funderburg NT,Jiang Y,Debanne SM,et al. Rosuvastatin reduces vascular inflammation and T cell and monocyte activation in HIV-infected subjects on antiretroviral therapy[J]. J Acquir Immune Defic Syndr,2015,68(4):396-404.
[9] 陈建昌,高国洁,李凤玲,等.瑞舒伐他汀对ApoE-/-小鼠动脉粥样斑块中凋亡相关蛋白的影响[J].中国老年学杂志,2015,35(17):4792-4795.
[10] 蒋小晶,闫亚非,陈新云,等.瑞舒伐他汀对急性冠脉综合征患者炎症和细胞因子以及心血管事件的影响[J].川北医学院学报,2015,30(3):366-369.
[11] Kwon HM,Lee YS,Bae HJ,et al. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke[J]. Stroke,2014,45(3):871-873.
[12] Ansari R,Mahta A,Mallack E,et al. Hyperhomocysteinemia and neurologic disorders:a review[J]. J Clin Neurol,2014,10(4):281-288.
[13] 梁宇,马琳娜,杨晓玲,等.MMP-9 DNA甲基化变化在ApoE-/-小鼠肾脏中的作用及高蛋氨酸饮食的影响[J].中国现代医学杂志,2012,22(32):7-12.
[14] 刘爱宁,杨文刚,鞠树红,等.不同剂量瑞舒伐他汀对实验性动脉粥样硬化性大鼠过氧化及血管平滑肌增生的影响[J].河北医科大学学报,2015,36(2):133-136.
[15] 鲍晓梅,郑宏超.阿托伐他汀对高同型半胱氨酸血症诱发动脉粥样硬化的干预作用[J].心脏杂志,2015,27(1):1-6.