肺癌在我国的发病率逐年上升,目前已经成为各类肿瘤之首。肺癌患者往往合并发热,发热原因可能是感染性疾病或者肿瘤因素。肺癌患者免疫力低、抵抗力差,容易并发感染,其中以细菌性感染为主,是导致其死亡的主要原因之一。合并发热的肺癌患者,由于骨髓抑制等因素,其中性粒细胞升高不明显,影响对感染性发热与肿瘤热的判断,不恰当的抗生素使用并不能使患者的病情得到缓解,还增加了医疗费用。另外,潜在的感染将会导致化疗的延迟。所以,化疗前发热的正确诊断至关重要。C反应蛋白(C-reactive protein,CRP)作为炎性指标在临床上已应用多年,但该指标的特异性较低[1]。最近,降钙素原(procalcitonin,PCT)作为细菌性感染的指标已应用于临床,其特异性要高于CRP[2-3]。本研究探讨CRP、PCT对非小细胞肺癌且中性粒细胞正常患者肿瘤热与感染性发热早期鉴别诊断的价值。报告如下。
1 资 料 与 方 法
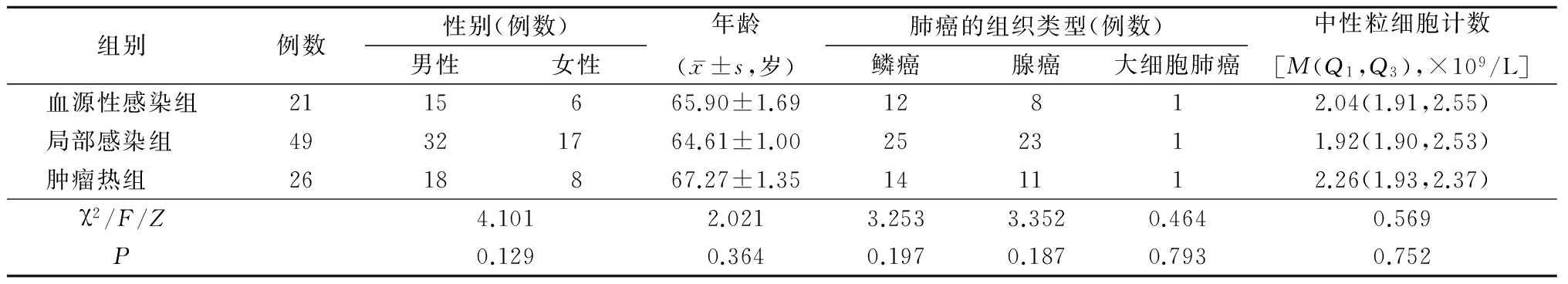
1.1一般资料 选择2015年9月—2017年9月河北医科大学第三医院呼吸内科收治的非小细胞肺癌合并发热患者96例,男性65例,女性31例,年龄52~80岁。所有患者经细胞学或病理学检查确诊并且肿瘤分期明确,均不存在中性粒细胞减少。选择腋下温度>37.5 ℃患者并将其分为3组。血源性感染组21例,血培养阳性;局部感染组49例,有局部感染的症状及相关实验室检查,主要包括肺炎、支气管炎、泌尿系感染;肿瘤热组26例,患者无临床感染的依据,无放射学及微生物学证据。从电子病历中分析96例患者的性别、年龄、合并疾病、肿瘤类型、PCT、CRP及中性粒细胞计数,其中PCT、CRP及中性粒细胞计数均为患者住院后次日清晨测定。3组之间一般资料差异无统计学意义(P>0.05),具有可比性,见表1。
表1 患者临床特点
Table1The clinical characteristics of the patients
1.2测定方法 采集所有肺癌发热患者血浆或血清标本,检测并记录PCT及CRP值。PCT测定采用免疫荧光分析法,CRP测定采用胶乳增强免疫比浊法,操作步骤均按照试剂盒或仪器的使用说明进行。结果判读:PCT正常参考值为<0.5 μg/L,若>0.5 μg/L则提示为细菌感染;CRP正常参考值为<8.0 mg/L,若>8.0 mg/L则提示存在细菌感染或其他急性时相反应。
1.3统计学方法 应用SPSS 16.0统计软件分析数据。非正态分布的计量资料以M(Q1,Q3)表示,组间比较采用秩和检验;正态分布的计量资料比较采用F检验;绘制受试者工作特征(receiver operating curve,ROC)曲线,并计算曲线下面积(area under curve,AUC),进而评价PCT、CRP、PCT/CRP在鉴别肿瘤热与感染性发热中的作用。P<0.05为差异有统计学意义。
2 结 果
2.13组PCT和CRP水平比较 血源性感染组和局部感染组PCT水平明显高于肿瘤热组(P<0.05);血源性感染组CRP水平明显高于肿瘤热组(P<0.05),但肿瘤热组与局部感染组差异无统计学意义(P>0.05);血源性感染组和局部感染组PCT/CRP比值明显高于肿瘤热组(P<0.05)。见表2。
表2 3组PCT、CRP及PCT/CRP水平比较
Table2The PCT,CRP and PCT/CRP level in tumor fever group,bloodstreaminfection group and localized bacterial infection group[M(Q1,Q3)]
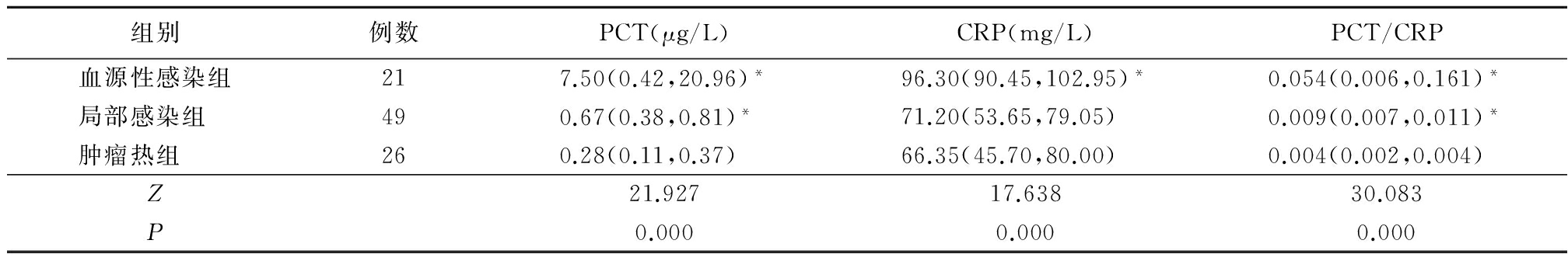
*P<0.05与肿瘤热组比较(秩和检验)
2.2PCT、CRP和PCT/CRP诊断感染价值的比较 绘制ROC曲线,以1-特异度为横坐标,敏感度为纵坐标。鉴别肿瘤热与局部感染时,通过曲线下面积得出PCT/CRP为最佳判断指标,其曲线下面积为0.809(95%CI=0.698~0.921),PCT曲线下面积为0.773(95%CI=0.659~0.887),CRP曲线下面积为0.544(95%CI=0.401~0.686);PCT/CRP最佳截断点为0.005 5,其对应的敏感度为81.6%,特异度为96.2%。鉴别肿瘤热与血源性感染时,通过曲线下面积得出PCT/CRP为最佳判断指标,其曲线下面积为0.901(95%CI=0.788~1.000),PCT曲线下面积为0.840(95%CI=0.715~0.964),CRP曲线下面积为0.786(95%CI=0.636~0.935);PCT/CRP的最佳截断点为0.005 0,其对应的敏感度为90.5%,特异度为92.3%。
3 讨 论
发热是肺癌患者常见的情况,其往往与感染因素或者非感染因素有关。为了尽快给予恰当的治疗,并且使病死率最小化,对于临床医生来说,尽快判断发热的原因至关重要。肿瘤热是一个除外诊断,因为没有临床特点作为肿瘤热的诊断标准[4],其常见症状是皮肤的发红及出汗,很少出现畏寒。相反,感染性发热往往表现因为周围血管扩张引起畏寒及发汗,革兰阴性杆菌引起的血源性感染往往表现为低血压及心动过速。感染是肺癌患者常见的并发症,可明显缩短患者的生存期。早期诊断肺癌患者的感染尤其重要,影像学资料还有微生物学检查作为感染的早期鉴别诊断是远远不够的,明确感染需要时间较长。为了鉴别肿瘤热与感染性发热,一些急性期的血液学标志物被研究,如PCT、CRP、血沉等,这些血液学指标结果时效性强,可以在1 h内测定完成。关于这些血液学指标在肿瘤发热患者中的鉴别作用是有争议的,有证据表明PCT能鉴别发热的原因[5-6]。在炎症、肿瘤、感染时CRP会增加[7],这使得CRP在鉴别肿瘤患者感染热与肿瘤热的特异性下降。
CRP是一种急性期蛋白,在炎症存在时,主要由肝细胞产生。在不同的急性或慢性疾病中,CRP经常作为炎性指标[7-8]。PCT是降钙素的前体蛋白,含有116个氨基酸,主要由甲状腺的C细胞或者神经内分泌细胞分泌。在细菌性或真菌性感染时,对抗内毒素和炎症因子反应性增高,其水平和感染的严重程度呈正相关。研究已经发现PCT在发热肿瘤患者的鉴别诊断中起着重要作用[9-10]。
中性粒细胞减少性发热是接受化学治疗的肿瘤患者常见的感染性并发症。研究证实,在中性粒细胞减少性发热的肿瘤患者中,PCT及CRP水平是明显增加的,并且是重要的生物学标志物[11-12]。到目前为止,对于中性粒细胞正常的非小细胞肺癌患者,关于PCT及CRP鉴别肿瘤热与感染的诊断价值尚未见报道。本研究显示肿瘤热组PCT水平明显低于局部感染组及血源性感染组。与Hangai等[13]报道的在血液恶性肿瘤中PCT的作用一致。但是Shomali等[14]研究发现在非中性粒细胞减少的肿瘤患者中,PCT水平在肿瘤热组与血源性感染组之间差异无统计学意义,原因可能为研究患者的肿瘤类型不同,其研究的肿瘤病例中包括实体瘤、淋巴瘤及多发性骨髓瘤;另外,肿瘤热组患者8例,样本量少,容易出现偏差。本研究中所有对象局限为非小细胞肺癌患者,并且样本量增加,在一定程度上避免了上述可能存在的偏差,但仍需大量样本研究进一步证实。本研究肿瘤热组CRP水平较血源性感染组明显减低,但与局部感染组CRP水平相比差异无统计学意义。Penel等[15]在肿瘤患者中研究发现肿瘤热组CRP水平与感染组CRP水平差异无统计学意义。也就是说在鉴别肿瘤患者感染性发热与肿瘤热方面,CRP预测价值不大。
已有研究证实,在诊断感染性疾病时,PCT的价值优于CRP[16-17]。在一项肿瘤晚期患者的临床研究中,结果显示肿瘤性发热组与细菌感染性发热组相比,感染组PCT明显升高,而CRP水平差异无统计学意义[18]。提示PCT在鉴别肿瘤患者肿瘤热与感染性发热方面有重要意义。Nath等[2]研究儿童恶性肿瘤合并感染的患者,测定CRP和PCT水平,结果显示PCT的ROC曲线下面积为0.751,CRP的曲线下面积为0.638,证实PCT在鉴别肿瘤合并感染患者时要优于CRP。本研究结果与其一致。
本研究ROC曲线下面积证实,在鉴别肿瘤热与感染性发热时,PCT作用优于CRP,PCT/CRP相比PCT、CRP,ROC曲线下面积最大,其作用更加显著。表明可以通过PCT/CRP的大小,早期对非小细胞肺癌患者中肿瘤热与感染性发热进行鉴别,以减少不必要的抗生素应用,从而避免医疗资源的浪费。Hahn等[19]在新生儿脓毒症的研究中发现,PCT/CRP曲线下面积为0.73,PCT为0.70,CRP为0.51,PCT/CRP在鉴别感染性发热方面比PCT、CRP更有优势。本研究结果与其一致。
本研究还存在一些不足之处:①本研究属于小样本回顾性分析研究,仍需要进一步进行大样本、前瞻性研究证实PCT、CRP及其比值的作用;②感染组中,不能完全排除肿瘤热与感染性发热共存的情况;③因为很多因素影响血培养的结果,有可能假阴性的血源性感染患者被包含在肿瘤热组。
总之,本研究证实,在鉴别肿瘤热与感染性发热时,PCT诊断价值优于CRP,PCT/CRP更优于PCT。
[参考文献]
[1] Ma L,Zhang H,Yin YL,et al. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome[J]. Cytokine,2016,88:126-135.
[2] Nath NS,Jayapalan S,Nair H,et al. Comparative diagnostic test evaluation of serum procalcitonin and C-reactive protein in suspected bloodstream infections in children with cancer[J]. J Med Microbiol,2017,66(5):622-627.
[3] Tanriverdi H,Tor MM,Kart L,et al. Prognostic value of serum procalcitonin and C-reactive protein levels in critically ill patients who developed ventilator-associated pneumonia[J]. Ann Thorac Med,2015,10(2):137-142.
[4] Pasikhova Y,Ludlow S,Baluch A. Fever in Patients With Cancer[J]. Cancer Control,2017,24(2):193-197.
[5] Durnas B,Watek M,Wollny T,et al. Utility of blood procalcitonin concentration in the management of cancer patients with infections[J]. Onco Targets Ther,2016,9:469-475.
[6] Hu L,Shi Q,Shi M,et al. Diagnostic Value of PCT and CRP for Detecting Serious Bacterial Infections in Patients With Fever of Unknown Origin:A Systematic Review and Meta-analysis[J]. Appl Immunohistochem Mol Morphol,2017,25(8):e61-69.
[7] Kiszewska N,Bien E,Irga-Jaworska N,et al. Selected inflammatory markers in the diagnosis and monitoring of infections in children treated for hematological malignancies[J]. Biomark Med,2015,9(5):461-471.
[8] 李晓华,吕巧云,温子海,等.老年糖尿病伴重症肺炎患者血清炎性反应及降钙素原变化的意义[J].河北医科大学学报,2016,37(3):316-318.
[9] Murat SA,Kose F,Taner SA,et al. Prognostic value of procalcitonin in infection-related mortality of cancer patients[J]. J BUON,2016,21(3):740-744.
[10] Hemming V,Jakes AD,Shenton G,et al. Prospective cohort study of procalcitonin levels in children with cancer presenting with febrile neutropenia[J]. BMC Pediatr,2017,17(1):2.
[11] Debiane L,Hachem RY,Al WI,et al. The utility of proadrenomedullin and procalcitonin in comparison to C-reactive protein as predictors of sepsis and bloodstream infections in critically ill patients with cancer[J]. Crit Care Med,2014,42(12):2500-2507.
[12] Thursky KA,Worth LJ. Can mortality of cancer patients with fever and neutropenia be improved?[J]. Curr Opin Infect Dis,2015,28(6):505-513.
[13] Hangai S,Nannya Y,Kurokawa M. Role of procalcitonin and C-reactive protein for discrimination between tumor fever and infection in patients with hematological diseases[J]. Leuk Lymphoma,2015,56(4):910-914.
[14] Shomali W,Hachem R,Chaftari AM,et al. Can procalcitonin distinguish infectious fever from tumor-related fever in non-neutropenic cancer patients?[J].Cancer,2012,118(23):5823-5829.
[15] Penel N,Fournier C,Clisant S,et al. Causes of fever and value of C-reactive protein and procalcitonin in differentiating infections from paraneoplastic fever[J]. Support Care Cancer,2004,12(8):593-598.
[16] Nishikawa H,Shirano M,Kasamatsu Y,et al. Comparative usefulness of inflammatory markers to indicate bacterial infection-analyzed according to blood culture results and related clinical factors[J]. Diagn Microbiol Infect Dis,2016,84(1):69-73.
[17] 朱康元,童武华,张青贵,等.降钙素原、C反应蛋白在细菌性肺炎诊断价值研究[J].国际呼吸杂志,2012,32(24):1844-1846.
[18] 陈银葵,邓欢,蔡思娜,等.肿瘤晚期患者感染与肿瘤热早期诊断的临床研究[J].中华医院感染学杂志,2016,26(12):2721-2723.
[19] Hahn WH,Song JH,Kim H,et al. Is procalcitonin to C-reactive protein ratio useful for the detection of late onset neonatal sepsis? [J]. J Matern Fetal Neonatal Med,2018,31(6):822-826.