血管病变是糖尿病最主要的并发症之一。在糖尿病血管病变的发生发展过程中,血管平滑肌细胞(vascular smooth muscle cells,VSMCs)发挥了重要作用。高血糖和内皮细胞释放的细胞因子作用于VSMCs,使之发生炎症反应和功能改变,进而导致血管病变的发生[1-4]。研究证明,锌指转录因子Krüppel样因子5(Krüppel-like factor 5,KLF5)在血管重塑中发挥重要作用[5]。在炎症因子刺激下,KLF5通过激活炎症反应信号通路加重炎症反应。核因子κB(nuclear factor κB,NF-κB)作为多能转录因子,可由多种信号(病毒/细菌感染、细胞因子、氧化应激等)诱导活化。NF-κB活化后,可进入细胞核与特定靶基因启动子结合,促进其进行转录,这些表达产物参与炎症反应、细胞增殖和免疫应答等过程[6-7]。体内和体外实验均证明,KLF5通过增加NF-κB p65的磷酸化促进炎症发生[8]。此外,佛波酯可增强VSMCs中KLF5和NF-κB p50的相互作用。但KLF5和NF-κB p50在高糖诱导的VSMCs炎症中的作用和相互关系尚不清楚。本研究旨在通过体外实验证实,高糖通过诱导KLF5表达及与NF-κB p50相互作用,进而促进VSMCs炎症的发生,报告如下。
1 材料与方法
1.1 细胞培养和试剂 细胞培养小鼠主动脉平滑肌细胞株购自American Type Culture Collection,上述细胞在含10%胎牛血清(fetal bovine serum,FBS)、105 U/L青霉素和10-7 g/L链霉素的DMEM低糖培养基,于37 ℃、含5% CO2中培养。待细胞生长至70%~80%密度时,进行传代。
1.2 试剂和仪器 葡萄糖(Sigma公司);兔抗KLF5多克隆抗体(GeneTex公司);鼠抗β-actin多克隆抗体(Santa Cruz公司);鼠抗NF-κB p105/p50单克隆抗体(Santa Cruz公司);鼠抗TNF-α单克隆抗体(Abcam公司);IgG一抗(Bioworld公司);Protein A-Agarose(Santa Cruz公司);lipofectamine 2000细胞转染试剂(Invitrogen公司);Opti-Mem培养基(Gibco公司);核酸定量仪(德国Eppendorf公司);PCR扩增仪(德国Eppendorf公司);实时荧光定量PCR仪ABI 7500 Fast(美国ABI公司);电泳仪(Bio-rad公司);ECL化学发光仪(VilberLourmat公司)。
1.3 引物设计与合成 上海生物工程公司合成,引物序列见表1。
表1 GAPDH,KLF5,NF-κB p105/p50,TNF-α,siKLF5 #1,siKLF5 #2 和siCtrl引物序列
Table 1 Primer sequences of GAPDH,KLF5,NF-κB p105/p50,TNF-α,siKLF5 #1,siKLF5 #2 and siCtrl
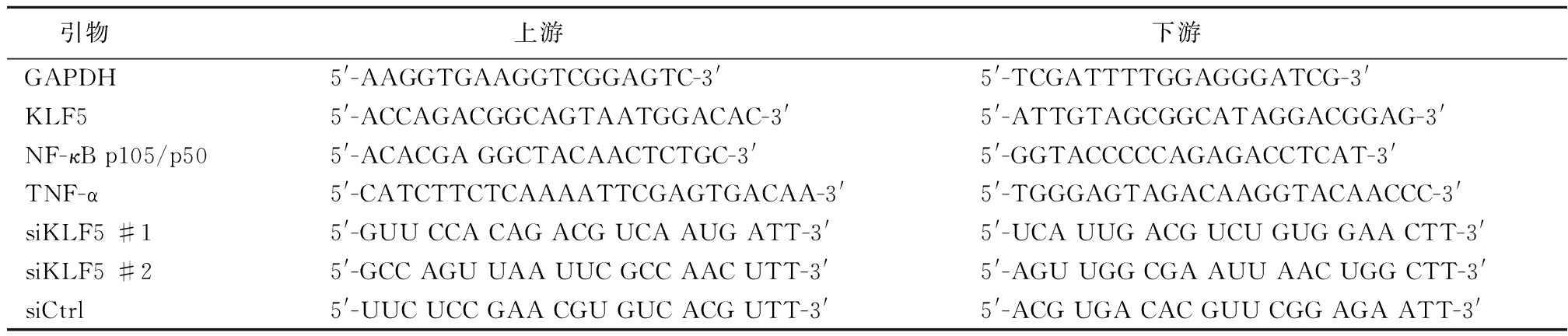
引物 上游 下游 GAPDH5'-AAGGTGAAGGTCGGAGTC-3'5'-TCGATTTTGGAGGGATCG-3'KLF55'-ACCAGACGGCAGTAATGGACAC-3'5'-ATTGTAGCGGCATAGGACGGAG-3'NF-κB p105/p505'-ACACGA GGCTACAACTCTGC-3'5'-GGTACCCCCAGAGACCTCAT-3'TNF-α5'-CATCTTCTCAAAATTCGAGTGACAA-3'5'-TGGGAGTAGACAAGGTACAACCC-3'siKLF5 #15'-GUU CCA CAG ACG UCA AUG ATT-3'5'-UCA UUG ACG UCU GUG GAA CTT-3'siKLF5 #25'-GCC AGU UAA UUC GCC AAC UTT-3'5'-AGU UGG CGA AUU AAC UGG CTT-3'siCtrl5'-UUC UCC GAA CGU GUC ACG UTT-3'5'-ACG UGA CAC GUU CGG AGA ATT-3'
1.4 细胞处理 用不同浓度葡萄糖(0~25 mmol/L)处理VSMCs 12 h或用高糖(25 mmol/L)处理VSMCs不同时间(0~12 h),收集细胞用于以下实验。
1.5 转染 应用Lipofectamine 2000对细胞转染,具体步骤参照Lipofectamine 2000 细胞转染试剂说明书。
1.6 RNA提取和实时定量PCR 采用Trizol法提取细胞总RNA,核酸定量仪检测RNA的纯度和浓度。按照Invitrogen公司“用于qRT-PCR的M-MLV第一链合成系统”操作说明,取1~3 μg总RNA建立20 μL逆转录体系合成cDNA。之后,用Invitrogen公司的“Platinum SYBR Green QpcrSuperMix-UDG with ROX”试剂盒和ABI7500 Fast Real-time PCR扩增仪进行荧光扩增。Real-time PCR反应结果分析:以GAPDH rRNA为内参,采用△Ct(Ct目的-Ct内参)法进行相对定量分析,以2-△Ct作为目的RNA的相对表达量。
1.7 蛋白质印迹法(Western blot)分析 收集细胞,提取总蛋白,采用改良的Lowry法进行蛋白定量。取等量蛋白样品进行SDS-聚丙烯酰胺凝胶电泳,电泳完毕,取出凝胶进行半干转膜。转膜完毕,取出PVDF膜,放置在含5%脱脂奶粉的TTBS封闭液中,于室温封闭2 h后,将封闭后的PVDF膜用TTBS适当涮洗,然后放入用一抗稀释液稀释的一抗中,4 ℃放置过夜。次日,取出PVDF膜用TTBS适当涮洗,将PVDF膜置入用适当TTBS稀释的化学发光二抗中,室温反应2 h,取出PVDF膜用TTBS适当涮洗。最后用化学发光仪检测抗体特异结合条带。
1.8 免疫共沉淀(co-immunoprecipitation,CoIP) 用RIPA裂解液裂解细胞总蛋白,收集上清,采用改良的Lowry法进行蛋白定量。取上清(约500 μg蛋白)与抗体、PMSF混合,IPH washing buffer(50 mmol/L Tris-HCl,pH 8.0,150 mmol/L NaCl,5 mmol/L EDTA,0.5% NP-40,0.1 mmol/L PMSF)补足体积至300 μL,4 ℃颠倒混匀后加入Protein A珠子,4 ℃颠倒混匀过夜。次日4 ℃离心后,收集蛋白A-抗原-抗体三元复合物,依次用IPH washing buffer洗涤,洗涤后用2×SDS loading buffer悬浮沉淀,煮沸后离心取上清进行Western blot分析。
1.9 统计学方法 应用SPSS 13.0统计软件处理数据。计量资料比较分别采用单因素方差分析和LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
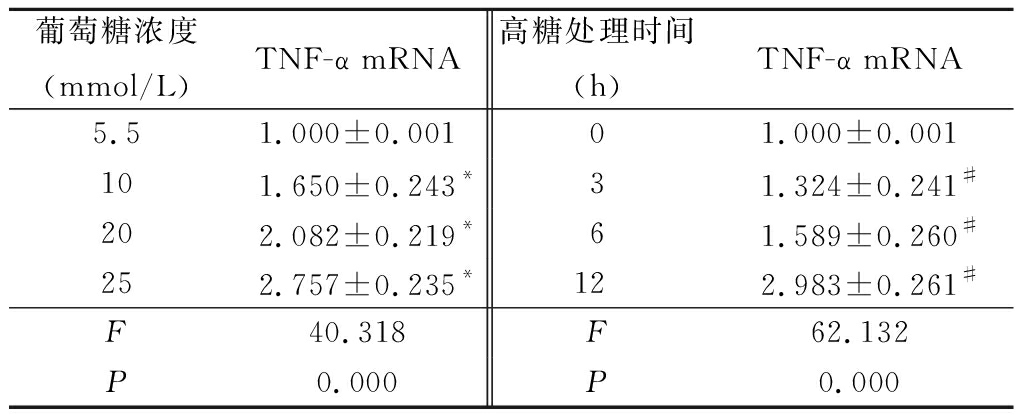
2.1 不同浓度葡萄糖和高糖处理VSMCs不同时间对TNF-α mRNA和蛋白的影响 体外培养的VSMCs用不同浓度葡萄糖(5.5~25 mmol/L)处理12 h,qRT-PCR结果显示,随着葡萄糖浓度的增加TNF-α mRNA表达随之增加,各浓度组与5.5 mmol/L浓度组比较,差异均有统计学意义(P<0.05); 而用高糖(25 mmol/L)处理VSMCs不同时间(0~12 h),qRT-PCR结果显示,随着处理时间的增加,TNF-α mRNA表达随之增加,各处理时间与0 h处理组时间比较,差异均有统计学意义(P<0.05)。Western blot结果显示,随着葡萄糖浓度的增加和高糖(25 mmol/L)处理时间的增加,TNF-α蛋白的表达随之增加。见表2,图1,2。
表2 不同浓度葡萄糖和高糖处理VSMCs不同时间对TNF-α基因表达的影响
Table 2 Effect of different doses of glucose or various times on mRNA expression of TNF-α in VSMCs![]()
葡萄糖浓度(mmol/L)TNF-α mRNA高糖处理时间(h)TNF-α mRNA5.51.000±0.00101.000±0.001101.650±0.243*31.324±0.241#202.082±0.219*61.589±0.260#252.757±0.235*122.983±0.261#F40.318F62.132P0.000P0.000
*P<0.05与5.5 mmol/L组比较 #P<0.05与0 h组比较(LSD-t检验)
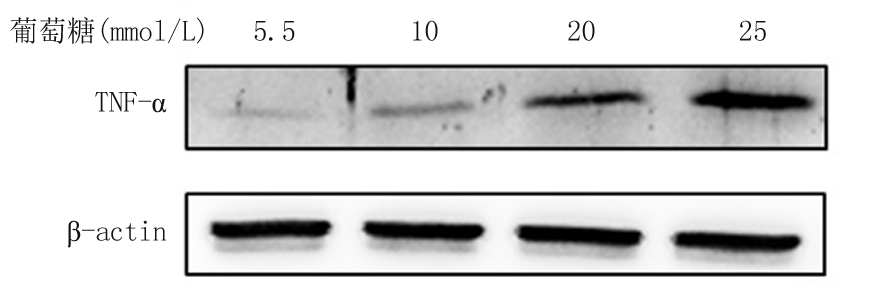
图1 不同浓度葡萄糖对VSMCs中TNF-α蛋白表达的影响
Figure 1 Effect of different doses of glucose on protein expression of TNF-α in VSMCs
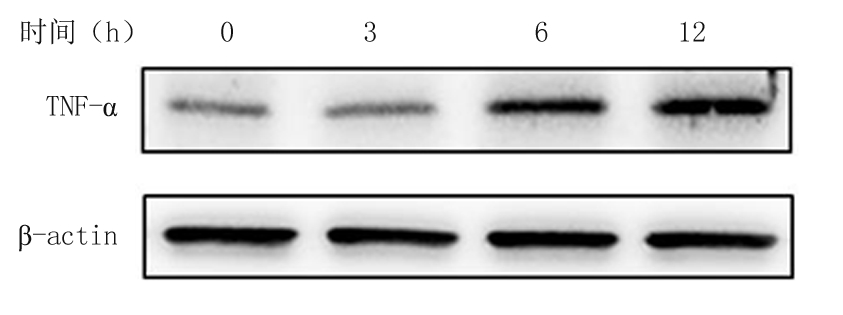
图2 高糖处理VSMCs不同时间对TNF-α蛋白表达的影响
Figure 2 Effect of various times on protein expressions of TNF-α in high glucose treated VSMCs
2.2 不同浓度葡萄糖和高糖处理VSMCs不同时间对KLF5和NF-κB p50的影响 体外培养的VSMCs用不同浓度葡萄糖(5.5~25 mmol/L)处理12 h,qRT-PCR结果显示,随着葡萄糖浓度的增加KLF5和NF-κB p50 mRNA的表达随之增加,各浓度组与5.5 mmol/L浓度组比较,差异均有统计学意义(P<0.05); 而用高糖(25 mmol/L)处理VSMCs不同时间(0~12 h),qRT-PCR结果显示,随着处理时间的增加,KLF5和NF-κB p50 mRNA的表达随之增加,各处理时间与0 h处理组比较,差异均有统计学意义(P<0.05)。Western blot结果显示,随着葡萄糖浓度的增加和高糖(25mmol/L)处理时间的增加,KLF5和NF-κB p50蛋白的表达随之增加,见表3,图3,4。
表3 不同浓度葡萄糖和高糖处理VSMCs不同时间KLF5、NF-κB p50基因表达的影响
Table 3 Effect of different doses of glucose or various times on mRNA expression of KLF5 and NF-κB p50 in VSMCs![]()
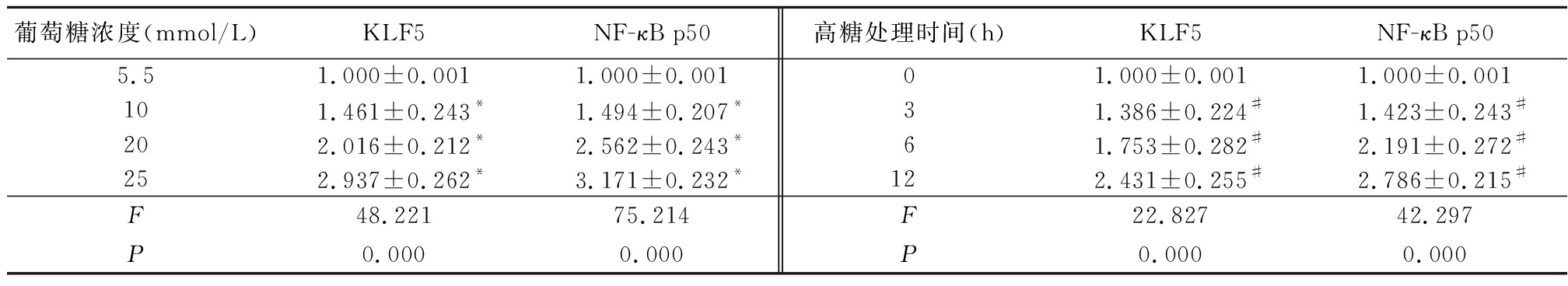
葡萄糖浓度(mmol/L)KLF5NF-κB p50高糖处理时间(h)KLF5NF-κB p505.51.000±0.0011.000±0.00101.000±0.0011.000±0.001101.461±0.243*1.494±0.207*31.386±0.224#1.423±0.243#202.016±0.212*2.562±0.243*61.753±0.282#2.191±0.272#252.937±0.262*3.171±0.232*122.431±0.255#2.786±0.215#F48.22175.214F22.82742.297P0.0000.000P0.0000.000
*P<0.05与5.5 mmol/L组比较 #P<0.05与0 h组比较(LSD-t检验)
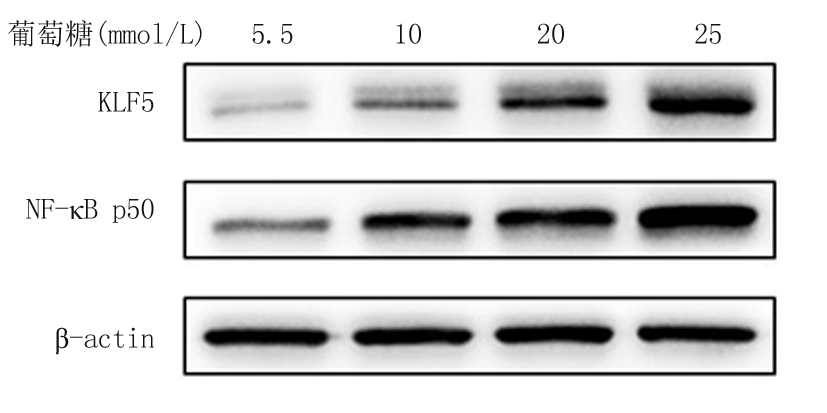
图3 不同浓度葡萄糖对VSMCs中KLF5、NF-κB p50蛋白表达的影响
Figure 3 Effect of different doses of glucose on protein expression of KLF5 and NF-κB p50 in VSMCs
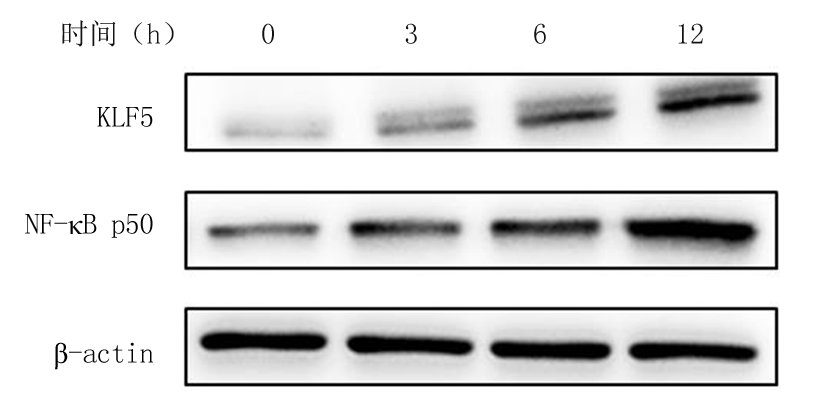
图4 高糖处理VSMCs不同时间对KLF5、NF-κB p50蛋白表达的影响
Figure 4 Effect of various times on protein expressions of KLF5 and NF-κB p50 in high glucose treated VSMCs
2.3 敲低KLF5后对TNF-α蛋白表达的影响 用siRNA将KLF5进行内源性敲低,Western blot结果显示,敲低KLF5后,无论是否给予高糖,TNF-α的表达均明显下调,而且si-KLF5+高糖组与si-Ctrl+高糖组相比,TNF-α蛋白的表达受到明显抑制,见图5。
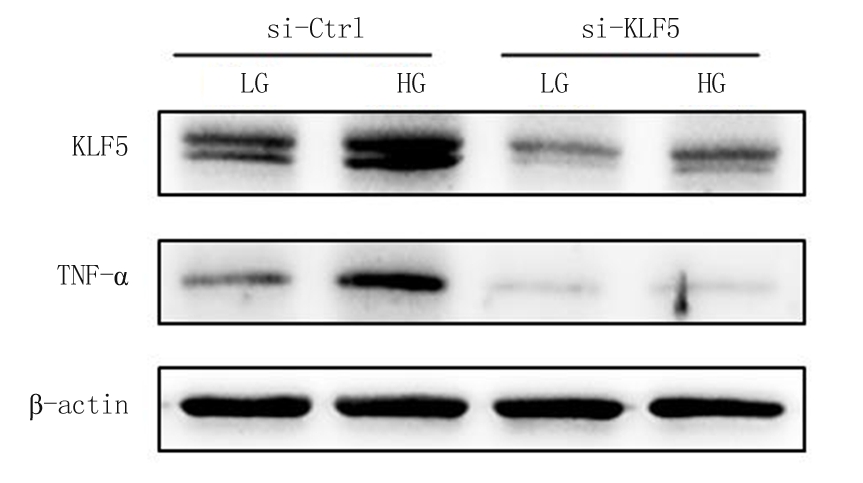
图5 内源性敲低KLF5后对TNF-α蛋白表达的影响
Figure 5 Effect of endogenous knockdown of KLF5 on the expression of TNF-α protein
2.4 高糖促进KLF5与NF-κB p50相互作用 为了进一步证明KLF5和NF-κB p50在高糖促进VSMCs炎症中的相互关系,采用CoIP的方法检测高糖处理VSMCs后KLF5和NF-κB p50的相互作用。结果显示,无论是低糖还是高糖处理VSMCs,KLF5均可被NF-κB p50抗体沉淀,同样,NF-κB p50可被KLF5抗体沉淀。高糖处理VSMCs后,高糖组与低糖组相比,KLF5和NF-κB p50的相互作用显著增强,见图6。
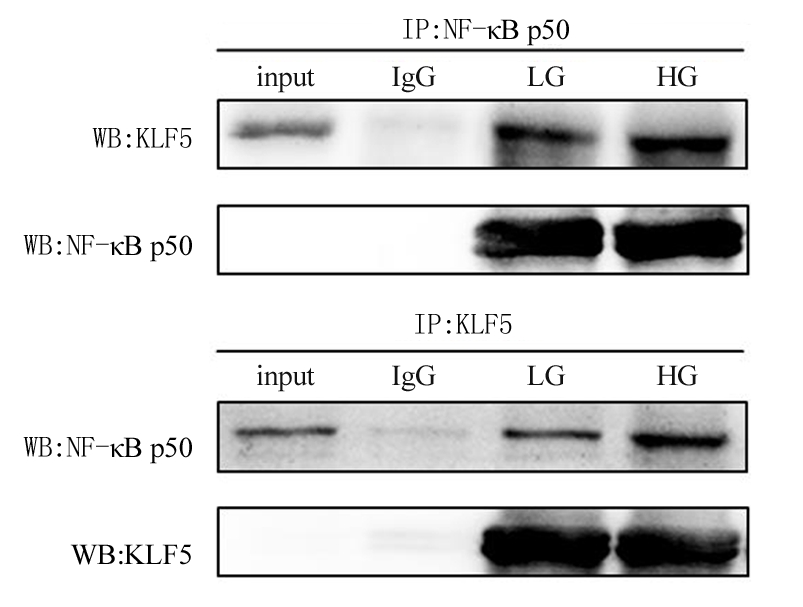
图6 高糖促进KLF5与NF-κB p50相互作用
Figure 6 High glucose promotes the interaction between KLF5 and NF-κB p50
3 讨 论
随着我国人民饮食结构的改变,糖尿病的发病率呈现逐年上升趋势,严重危害人民健康。糖尿病对机体的危害主要来自高血糖导致的多种并发症,其中以心血管系统最容易受累[9-10]。在2型糖尿病血管病变的发生发展过程中,高血糖是重要的危险因素[11-13]。高糖诱发的炎症和氧化应激导致血管内皮功能受损,并诱导内皮细胞表达多种促炎细胞因子,促进血管炎症发生[14]。VSMCs是血管壁的重要组成部分。当有高血糖和(或)细胞因子作用时,VSMCs可发生炎症反应和功能改变,在糖尿病血管病变的发生发展过程中发挥重要作用。本研究以体外培养的VSMCs为研究对象,研究了高糖对炎症基因TNF-α表达的影响,结果显示高糖上调VSMCs中TNF-α的表达,呈浓度及时间依赖性。与先前研究结果一致[15]。
KLF是含锌指结构的转录因子家族,在细胞增殖、分化和凋亡等过程中发挥重要作用。KLF5与细胞增殖、肿瘤发生和胚胎发育密切相关[16-19]。研究发现,在动脉粥样硬化的发生过程中,新生血管内膜VSMCs中KLF5表达显著增加[20]。
作为多能性转录因子的NF-κB,被氧化应激、细胞因子、细菌/病毒感染等多种信号诱导活化后,进入细胞核与靶基因启动子结合并促进其转录。有研究表明,异常活化的NF-κB可加重炎症反应,同时也可以引起免疫应答异常。在糖尿病并发症的发生发展过程中,NF-κB发挥重要作用。有研究发现,肠道上皮细胞中,KLF5通过促进NF-κB磷酸化上调脂多糖诱导的促炎细胞因子表达[8]。但糖尿病大血管病变时,血糖处理VSMCs对KLF5和NF-κB p50两者相互关系的影响尚不清楚。为了明确在高糖诱导的VSMCs炎症中,KLF5和NF-κB p50是否发挥作用,本研究检测了高糖对KLF5和NF-κB p50表达水平的影响,结果显示在mRNA和蛋白水平高糖诱导KLF5和NF-κB p50 表达,呈浓度及时间依赖性。在高糖刺激下,VSMCs中KLF5和NF-κB p50 表达上调的同时,炎症因子表达也上调。为了探讨KLF5在高糖诱导VSMCs炎症中的作用,本研究用siRNA内源性敲低KLF5,结果显示敲低KLF5后,高糖诱导的VSMCs炎症受到明显抑制,表明高糖的促炎症效应依赖于KLF5的作用。有研究表明,佛波酯可增强VSMCs中KLF5和NF-κB p50的相互作用,1,25-(OH)2D3阻抑巨噬细胞中KLF5与NF-κB p50的相互作用,进而发挥抗炎和抗增殖的作用[21]。为了探明KLF5与NF-κB p50是以何种方式协同调节高糖诱导的VSMCs炎症,本研究用CoIP的方法证明了KLF5和NF-κB p50以蛋白质-蛋白质相互作用的形式发挥作用,二者协同介导VSMCs的炎症。
总之,本研究从蛋白质-蛋白质相互作用的角度,证明了KLF5和NF-κB p50通过相互作用调节高糖诱导的VSMCs炎症。本研究为揭示高糖诱导血管炎症的分子机制提供了新的理论依据。
[1] Yang J,Chen L,Ding J,et al. MicroRNA-24 inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1[J]. Gene,2016,586(2):268-273.
[2] Sun YY,Qin SS,Cheng YH,et al. MicroRNA expression profile and functional analysis reveal their roles in contact inhibition and its disruption switch of rat vascular smooth muscle cells[J]. Acta Pharmacol Sin,2018,39(5):885-892.
[3] Reddy MA,Das S,Zhuo C,et al. Regulation of Vascular Smooth Muscle Cell Dysfunction Under Diabetic Conditions by miR-504[J]. Arterioscler Thromb Vasc Biol,2016,36(5):864-873.
[4] Bennett MR,Sinha S,Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis[J]. Circ Res,2016,118(4):692-702.
[5] Zheng B,Zheng CY,Zhang Y,et al. Regulatory crosstalk between KLF5,miR-29a and Fbw7/CDC4 cooperatively promotes atherosclerotic development[J]. Biochim Biophys Acta,2018,1864(2):374-386.
[6] Sun X,Zeng H,Wang Q,et al. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-kB pathway[J]. Exp Cell Res,2018,369(1):112-119.
[7] Ye Y,Bian W,Fu F,et al. Selenoprotein S inhibits inflammation-induced vascular smooth muscle cell calcification[J]. J Biol Inorg Chem,2018,23(5):739-751.
[8] Chen HL,Chong IW,Lee YC,et al. Kruppel-like factor 5 mediates proinflammatory cytokine expression in lipopolysaccharide-induced acute lung injury through upregulation of nuclear factor-κB phosphorylation in vitro and in vivo[J]. Mediators Inflamm,2014,2014:281984.
[9] 杨保军,张丽平,殷洪山,等.冠心病与糖代谢异常关系临床调查分析[J].河北医科大学学报,2017,38(7):819-821.
[10] 刘晓丹,李琰.糖代谢异常与心血管疾病[J].中华糖尿病杂志,2016,24(3):132-134.
[11] 李兰英.2型糖尿病的影响因素及研究进展[J].河北医科大学学报,2013,34(9):1100-1102.
[12] Chen M,Zhang Y,Li W,et al. MicroRNA-145 alleviates high glucose-induced proliferation and migration of vascular smooth muscle cells through targeting ROCK1[J]. Biomed Pharmacother,2018,99:81-86.
[13] Wang K,Deng X,Shen Z,et al. High glucose promotes vascular smooth muscle cell proliferation by upregulating proto-oncogene serine/threonine-protein kinase Pim-1 expression[J]. Oncotarget,2017,8(51):88320-88331.
[14] 郑秋兰,胡晓光,李亚永.2型糖尿病下肢血管病变患者介入前后血脂和血清高敏C反应蛋白的变化[J].河北医科大学学报,2015,36(7):764-767.
[15] Hui Y,Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-κB signaling[J]. Life Sci,2018,207:212-218.
[16] Chen Z,Zhang Q,Wang H,et al. Klf5 mediates odontoblastic differentiation through regulating dentin-specific extracellular matrix gene expression during mouse tooth development[J]. Sci Rep,2017,7:46746.
[17] Ma D,Chang LY,Zhao S,et al. KLF5 promotes cervical cancer proliferation,migration and invasion in a manner partly dependent on TNFRSF11a expression[J]. Sci Rep,2017,7(1):15683.
[18] He P,Yang JW,Yang VW,et al. Krüppel-like factor 5,increased in pancreatic ductal adenocarcinoma,promotes proliferation,acinar-to-ductal metaplasia,pancreatic intraepithelial neoplasia,and tumor growth in mice[J]. Gastroenterology,2018,154(5):1494-1508.
[19] Azami T,Waku T,Matsumoto K,et al. Klf5 maintains the balance of primitive endoderm versus epiblast specification during mouse embryonic development by suppression of Fgf4[J]. Development,2017,144(20):3706-3718.
[20] Zhang XH,Zheng B,Yang Z,et al. TMEM16A and myocardin form a positive feedback loop that is disrupted by KLF5 during Ang Ⅱ-induced vascular remodeling[J].Hypertension,2015,66(2):412-421.
[21] Ma D,Zhang RN,Wen Y,et al. 1,25(OH)2D3-induced interaction of vitamin D receptor with p50 subunit of NF-κB suppresses the interaction between KLF5 and p50,contributing to inhibition of LPS-induced macrophage proliferation[J]. Biochem Biophys Res Commun,2017,482(2):366-374.