甲状腺功能亢进症(简称甲亢)是常见的内分泌系统疾病,好发于育龄期女性。国内报道妊娠期甲亢的发病率为0.1%~0.2%[1],而国外报道其在孕妇中的发病率为0.1%~0.4%[2]。妊娠合并甲亢引起流产、早产、宫内生长迟缓等发生率明显增高。氧化应激、缺血缺氧等均可激活转化生长因子β1(transforming growth factor β1,TGF-β1)及核因子κB (nuclear factor kappa B,NF-κB )信号转导通路,使肾间质纤维化,从而导致肾损伤[3-5]。本研究采用连续给予大鼠不同剂量的左甲状腺素钠灌胃的方法复制甲亢模型,甲亢模型制造成功后,母鼠可以自然受孕,之后继续给予左甲状腺素钠灌胃,制造妊娠合并甲亢的模型,旨在探讨母鼠妊娠合并甲亢与仔鼠肾间质纤维化的关系,以及随着仔鼠的生长发育,肾损伤的修复情况。
1 材料与方法
1.1 动物分组及模型建立 60日龄健康SD大鼠54只,其中雌鼠36只,雄鼠18只,体重(200±20)g,均购于河北省实验动物中心。饲养环境:室温18~25 ℃,相对湿度50%~70%,通风良好,光照与黑暗每12 h更替(模拟正常昼夜光线情况)。随机分为3组各18只,均给予普通饲料喂养。轻度甲亢组大鼠按每只每日50 μg/100 g的左甲状腺素钠溶于10 mL生理盐水中灌胃。重度甲亢组大鼠按每日每只150 μg /100 g左甲状腺素钠溶于10 mL生理盐水中灌胃。正常对照组大鼠每日给予10 mL生理盐水灌胃。尾静脉取血测定血清游离三碘甲状腺原氨酸(free tri-iodothyronine,FT3)、游离甲状腺素(free thyroxine,FT4)及促甲状腺素(thyroid stimulating hormone,TSH)。造模成功后按雌雄比例2∶1,与雄性SD大鼠合笼。大鼠受孕后继续灌胃,直至分娩。
1.2 试剂 血清 TSH、 FT3和 FT4 试剂盒均由Siemens Healthcare Diagnostics Products Limited提供;L-T4(商品名:优甲乐),德国默克公司产品;鼠抗鼠NF-κB P65抗体由SANTA CRUZ BITECHNOLOGY提供;兔抗鼠TGF-β1多克隆抗体由北京索莱宝科技有限公司提供。
1.3 方法
1.3.1 取材 待大鼠分娩后,各组分别于仔鼠0 d、21 d、60 d随机选取18只雄性仔鼠宰杀(避免雌激素及孕激素对甲状腺激素的影响),并分别标记为正常对照仔鼠组,轻度甲亢仔鼠组和重度甲亢仔鼠组。通过心脏采血法取21 d和60 d仔鼠血液,检测FT3、FT4、TSH。尿素氮(blood urea nitrogen,BUN)及血清肌酐(serum creatinine,SCr)。
1.3.2 肾小管间质TGF-β1及胞核NF-κB P65的测定 取左侧肾组织采用Western blot法测定肾小管间质细胞TGF-β1及胞核NF-κB P65的含量。
1.3.3 肾脏透射电镜 动物处死后,迅速取出右侧肾组织,在冰块上用锋利刀片切取1 mm×1 mm×1 mm肾皮质,4%戊二醛固定,1/15 mol/L磷酸缓冲液冲洗,1%四氧化锇后固定,1/15 mol/L磷酸缓冲液冲洗。经各梯度丙酮脱水。纯包埋液浸透后,将样品包埋在包埋板里。置入烤箱烘干后,用超薄切片机将样品切成厚约50 nm 的超薄切片,醋酸双氧铀及柠檬酸铅经行双重染色,透射电镜观察照相。
1.4 统计学方法 应用SPSS 15.0统计软件处理数据。计量资料比较分别采用F检验、SNK-q检验和配对t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 3组母鼠血清FT3、FT4及TSH比较 轻度甲亢母鼠组和重度甲亢母鼠组血清FT3、FT4均明显高于正常对照母鼠组,重度甲亢母鼠组又高于轻度甲亢母鼠组(P<0.05);轻度甲亢母鼠组和重度甲亢母鼠组血清TSH均明显低于于正常对照母鼠组,重度甲亢母鼠组又低于轻度甲亢母鼠组(P<0.05)。见表1。
2.2 3组仔鼠21 d及60 d时血清FT3、FT4及TSH比较 重度甲亢仔鼠组21 d 和60 d血清FT3、FT4均高于正常对照仔鼠组和轻度甲亢仔鼠组,各组60 d血清FT3、FT4均高于21 d(P<0.05);重度甲亢仔鼠组21 d 和60 d血清TSH均低于正常对照仔鼠组和轻度甲亢仔鼠组,各组60 d血清TSH均低于21 d(P<0.05)。见表2。
表1 3组母鼠甲状腺功能比较
Table 1 Comparison of thyroid function in three groups of female rats ![]()
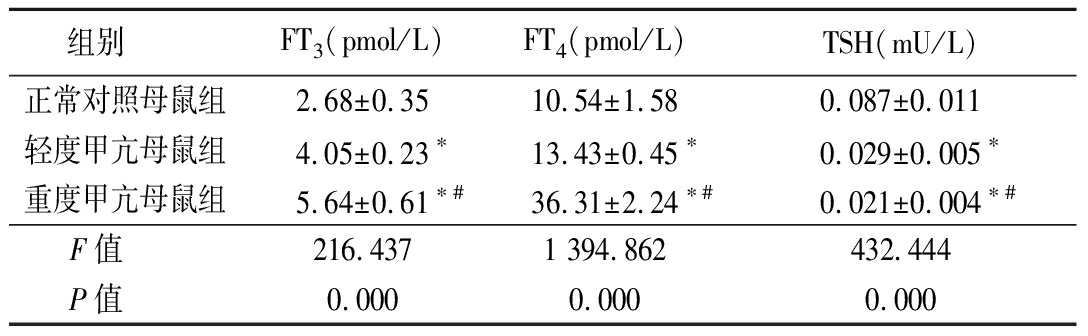
组别 FT3 (pmol/L)FT4 (pmol/L)TSH(mU/L)正常对照母鼠组2.68±0.3510.54±1.580.087±0.011轻度甲亢母鼠组4.05±0.23∗13.43±0.45∗0.029±0.005∗重度甲亢母鼠组5.64±0.61∗#36.31±2.24∗#0.021±0.004∗#F值 216.4371 394.862432.444P值 0.0000.0000.000
*P值<0.05与正常对照母鼠组比较 #P值<0.05与轻度甲亢母鼠组比较(SNK-q检验)
表2 3组仔鼠甲状腺功能比较
Table 2 Comparison of thyroid function in three groups of newborn rats ![]()
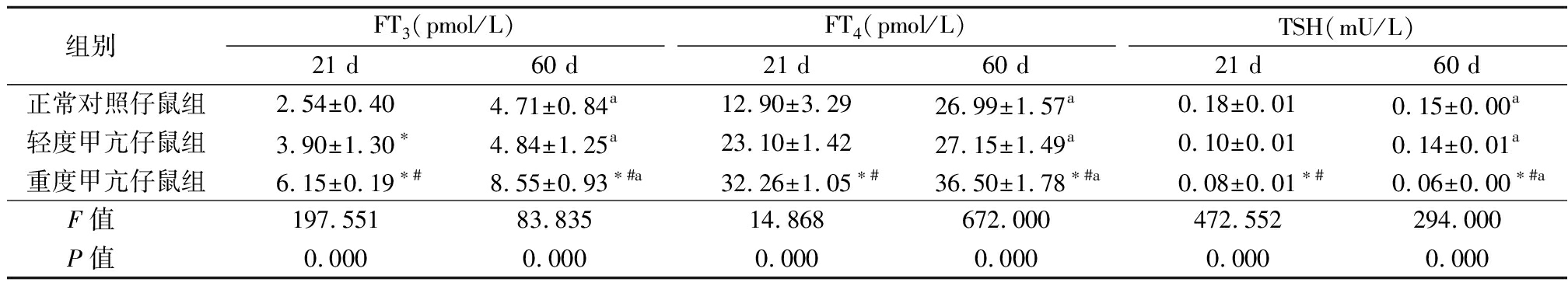
组别 FT3(pmol/L)21 d60 dFT4(pmol/L)21 d60 dTSH(mU/L)21 d60 d正常对照仔鼠组2.54±0.404.71±0.84a12.90±3.2926.99±1.57a0.18±0.010.15±0.00a轻度甲亢仔鼠组3.90±1.30∗4.84±1.25a23.10±1.4227.15±1.49a0.10±0.010.14±0.01a重度甲亢仔鼠组6.15±0.19∗#8.55±0.93∗#a32.26±1.05∗#36.50±1.78∗#a0.08±0.01∗#0.06±0.00∗#aF值 197.55183.83514.868672.000472.552294.000P值 0.0000.0000.0000.0000.0000.000
*P值<0.05与正常对照仔鼠组比较 #P值<0.05与轻度甲亢仔鼠组比较(SNK-q检验) aP值<0.05与21 d比较(配对t检验)
2.3 3组仔鼠21 d和60 d血清SCr和BUN水平比较 重度甲亢仔鼠组21 d 和60 d血清BUN和SCr均高于正常对照仔鼠组和轻度甲亢仔鼠组(P<0.05);60 d 时,轻度甲亢仔鼠组和重度甲亢仔鼠组BUN和SCr均高于21 d(P<0.05),正常对照仔鼠组60 d与21 d比较无明显变化(P>0.05)。见表3。
表3 3组仔鼠肾功能比较
Table 3 Comparison of renal function in three groups of newborn rats ![]()
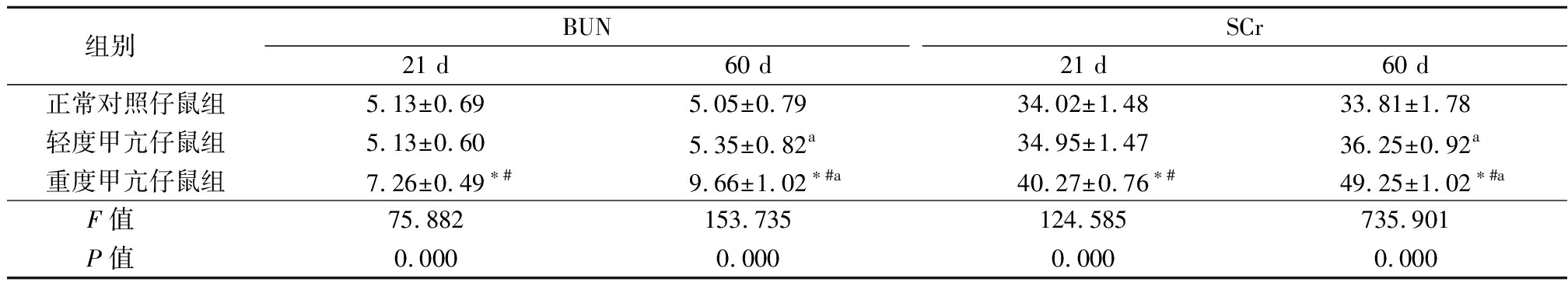
组别 BUN21 d60 dSCr21 d60 d正常对照仔鼠组5.13±0.695.05±0.7934.02±1.4833.81±1.78轻度甲亢仔鼠组5.13±0.605.35±0.82a34.95±1.47 36.25±0.92a重度甲亢仔鼠组7.26±0.49∗#9.66±1.02∗#a40.27±0.76∗#49.25±1.02∗#aF值 75.882153.735124.585735.901P值 0.0000.0000.0000.000
*P值<0.05与正常对照仔鼠组比较 #P值<0.05与轻度甲亢仔鼠组比较(SNK-q检验) aP值<0.05与21 d比较(配对t检验)
2.4 3组仔鼠肾小管间质细胞TGF-β1及NF-κB P65表达 轻度甲亢仔鼠组和重度甲亢仔鼠组0 d、21 d和60 d TGF-β1和NF-κB P65均高于正常对照仔鼠组,重度甲亢仔鼠组又高于轻度甲亢仔鼠组(P<0.05);正常对照仔鼠组21 d和60 d TGF-β1和NF-κB P65均高于0 d, 轻度甲亢仔鼠组和重度甲亢仔鼠组21 d和60 d TGF-β1和NF-κB P65均高于0 d,重度甲亢仔鼠组和轻度甲亢仔鼠组60 d又高于21 d(P<0.05)。见表4,图1~2。
表4 3组仔鼠肾小管间质细胞TGF-β1及NF-κB P65表达比较
Table 4 Comparison of TGF-β1 and NF-κB P65 in three groups of newborn rats ![]()
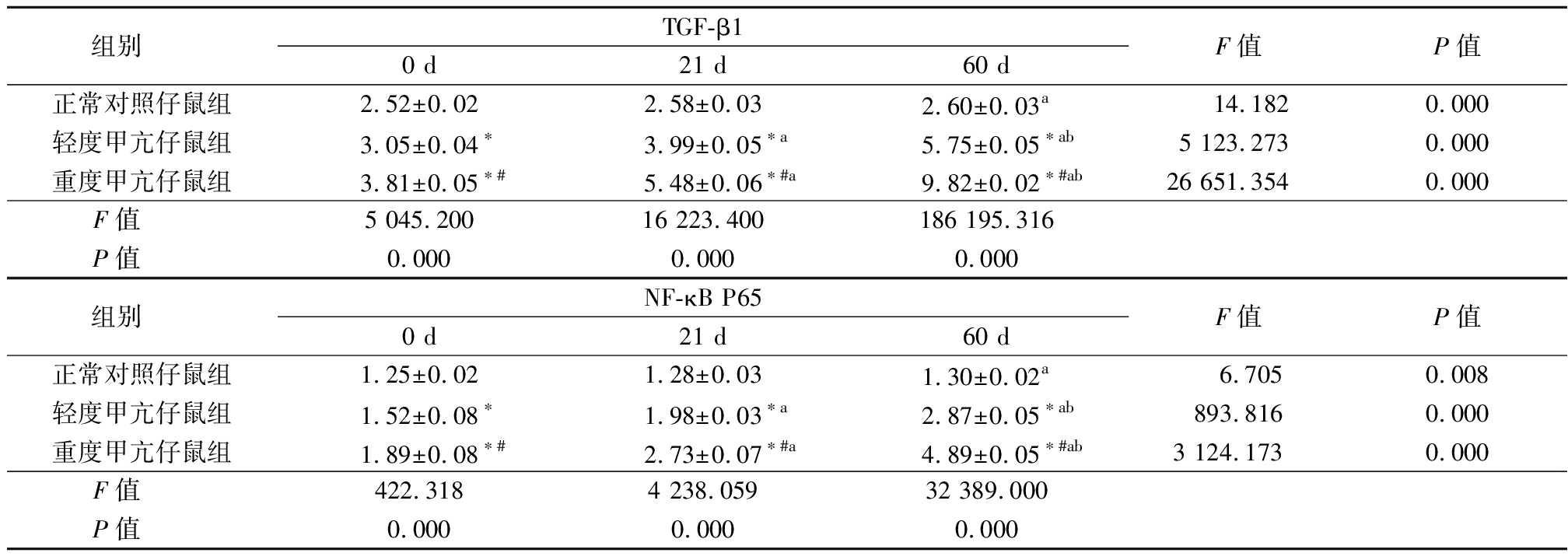
组别 TGF-β10 d21 d60 dF值P值正常对照仔鼠组2.52±0.022.58±0.032.60±0.03a 14.1820.000轻度甲亢仔鼠组3.05±0.04∗3.99±0.05∗a5.75±0.05∗ab5 123.2730.000重度甲亢仔鼠组3.81±0.05∗#5.48±0.06∗#a9.82±0.02∗#ab26 651.3540.000F值 5 045.20016 223.400186 195.316P值 0.0000.0000.000组别 NF-κB P650 d21 d60 dF值P值正常对照仔鼠组1.25±0.021.28±0.031.30±0.02a 6.7050.008轻度甲亢仔鼠组1.52±0.08∗1.98±0.03∗a2.87±0.05∗ab893.8160.000重度甲亢仔鼠组1.89±0.08∗#2.73±0.07∗#a4.89±0.05∗#ab3 124.1730.000F值 422.3184 238.05932 389.000P值 0.0000.0000.000
*P值<0.05与正常对照仔鼠组比较 #P值<0.05与轻度甲亢仔鼠组比较 aP值<0.05与0 d比较 bP值<0.05与21 d比较(SNK-q检验)
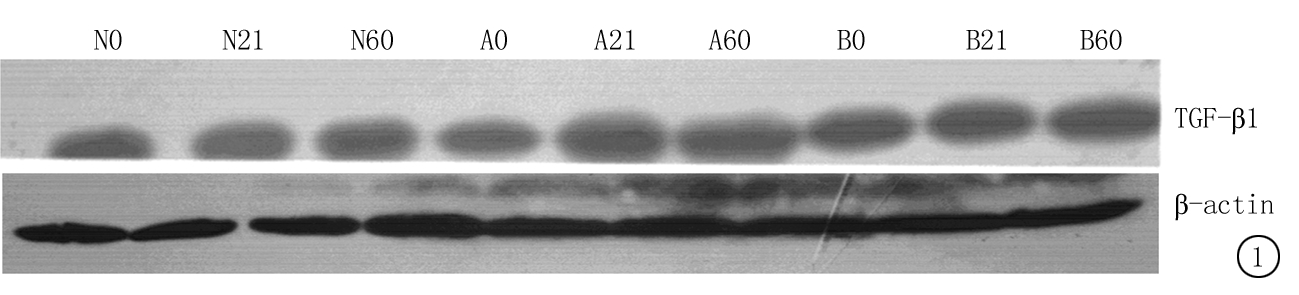
图1 各仔鼠组肾小管间质细胞TGF-β1表达
Figure 1 Expression of TGF-β1 in nucleus of renal tubular interstitial cell in three groups of newborn rats
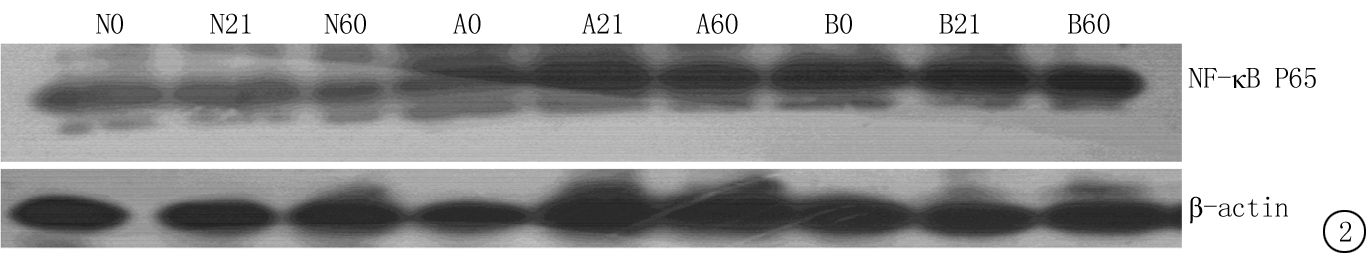
图2 各仔鼠组肾小管间质细胞胞核NF-κB P65表达
Figure 2 Expression of NF-κ B P65 in nucleus of renal interstitial cell in three groups of newborn rats
2.5 各组仔鼠21d时肾脏透射电镜结果 正常对照仔鼠组肾小球足细胞结构排列完整,足突排列清晰整齐;轻度甲亢仔鼠组肾小球基底膜轻度增厚,且厚薄不均匀,毛细血管窗孔不均匀增大;重度甲亢仔鼠组肾脏足细胞明显肿胀,足细胞线粒体鞘部分或大部分融合,部分膜融合或消失,系膜细胞和内皮细胞肿胀明显,系膜基质增多,毛细血管孔窗径明显增加。见图3。
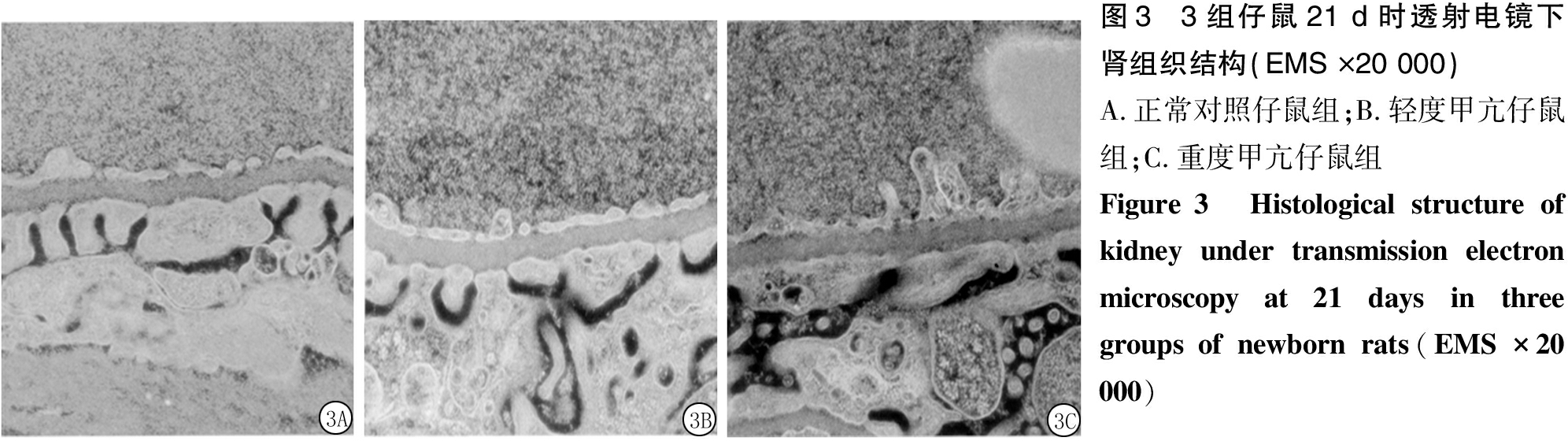
图3 3组仔鼠21 d时透射电镜下肾组织结构(EMS ×20 000)A.正常对照仔鼠组;B.轻度甲亢仔鼠组;C.重度甲亢仔鼠组Figure 3 Histological structure of kidney under transmission electron microscopy at 21 days in three groups of newborn rats(EMS ×20 000)
3 讨 论
本研究采用连续给予大鼠不同剂量左甲状腺素钠灌胃的方法复制甲亢模型,随着左甲状腺素钠剂量的增加,母鼠血清中FT3、FT4水平呈现升高趋势而TSH水平呈现下降趋势,3组间差异均有统计学意义(P<0.05);甲亢模型制造成功后,母鼠可以自然受孕,后继续给予左甲状腺素钠灌胃,制造甲亢合并妊娠的模型。与文献报道一致[6-7]。
妊娠合并甲亢会引起流产、早产、宫内生长迟缓等发生率明显增高。妊娠期间,甲状腺激素可通过胎盘进入胎儿体内[8]。随着时间的推移,仔鼠甲状腺不断发育,可分泌少量的甲状腺激素。本研究结果显示,21 d时轻度甲亢仔鼠组和重度甲亢仔鼠组FT3水平与正常对照仔鼠组差异均有统计学意义;由于仔鼠自身甲状腺不断发育,甲状腺激素在一定程度上得到补充,60 d时轻度甲亢仔鼠组与正常对照仔鼠组FT3、FT4及TSH差异无统计学意义。表明重度甲亢仔鼠组甲状腺发育受到严重影响,故与轻度甲亢仔鼠组及正常对照仔鼠组之间差异仍均有统计学意义。
研究表明,TGF-β1通过与细胞表面表皮生长因子受体结合,激发受体内在的酪氨酸激酶的活性,从而激活TGF-β/Smad信号通路:TβRⅡ和TGF-β配体结合后,激活TβRⅠ;TβRⅠ再磷酸化激活R-Smads(Smad1,2,3,4,5,8)某个成员,该成员再与Co-Smads(Smad4)结合后形成复合物转入核内,激活的复合物和两类DNA结合辅助因子(共抑制子及共激活子),决定靶基因的转录活性。该通路主要经过2种方式调控靶基因的转录:①Smad复合体入细胞核以后与其他转录因子结合形成稳定的复合物,间接地对目的基因进行调节;②Smad复合体入胞核后可直接与DNA结合,激活目的基因的表达[9-12]。
NF-κB广泛存在与各种哺乳动物的细胞中,是由Rel蛋白质家族中2种蛋白质分子组成的二聚体。其最主要发挥功能的形式为P50-P65组成的二聚体,正常情况下,NF-κB与IκB特异性结合,形成NF-κB/IκB存在于细胞质中。甲亢可导致机体氧化应激,导致NF-κB/IκB复合物分离,NF-κB进入细胞核中,并与靶基因启动子区域内的κB位点特异性结合,从而调控靶基因的转录[13-15]。
肾间质纤维化是许多慢性肾脏疾病进展为终末期肾衰竭的共同途径,是独立于病因之外的进展过程。肾间质纤维化是以肾小管的萎缩,间质成纤维细胞增生和细胞外基质过度沉积为主要特征的病理生理过程。
甲亢可以引起新生儿早产,早产儿肾脏发育不全,引起肾单位先天性数目减少。肾单位数目减少导致残余肾小球代偿性增大,随着肾小球体积增大,足细胞覆盖面积将会增大,足细胞的结构及功能发生变化,导致基底膜裸露和足细胞的脱落,肾小球的血管袢和肾小囊发生粘连,引起肾小球硬化[16]。研究表明,甲亢引起的氧化应激可导致肾间质纤维化。通过TGF-β/Smad及TGF-β/NF-κB信号通路,导致肾脏细胞的坏死、凋亡及肾脏间质组织的成纤维细胞增生,肾脏纤维化,基底膜增厚,导致肾小球硬化,同时肾脏入球小动脉的内皮损伤,肾滤过屏障遭到破坏,导致尿蛋白的增多。损伤的足细胞既可因为结构异常而导致肾功能改变,加重了肾损害的进程;又可因为其自身也同时发生表型转化为纤维样细胞,直接或间接通过产生某些促细胞外基质,生成因子,促使细胞外基质积聚,从而最终导致肾组织硬化和纤维化[17]。
本研究正常对照仔鼠组0 d及21 d比较,TGF-β1及NF-κB P65水平无明显差异,而轻度甲亢仔鼠组0 d及21 d时TGF-β1及NF-κB P65水平差异有统计学意义,重度度甲亢仔鼠组0 d及21 d时TGF-β1及NF-κB P65水平差异也有统计学意义。表明子代甲状腺功能的异常与肾间质纤维化有关。
本研究轻度甲亢仔鼠组肾间质细胞TGF-β1及NF-κB P65表达水平在同期明显高于正常对照仔鼠组,重度甲组仔鼠组肾间质细胞TGF-β1及NF-κB P65表达水平又明显高于同期轻度甲亢仔鼠组。表明不同的甲状腺激素水平对子代肾间质纤维化是不尽相同的;母亲甲状腺激素水平越高,同时期子代肾间质纤维化程度越重。
综上所述,母体甲亢可引起子代肾间质纤维化,随着母体甲亢程度的加重,子代肾间质纤维化的程度更加明显。且随着子代的生长发育,肾间质纤维化的程度更加明显。因此,甲亢的早期诊断与治疗对预防子代肾间质纤维化具有重要意义,对优生优育至关重要。
[1] 孙浩国.妊娠及产后甲状腺毒症诊治进展[J].中国冶金工业医学杂志,2017,34(1):20-21.
[2] Wang Y,Sun XL,Wang CL,et al. Influence of screening and intervention of hyperthyroidism on pregnancy outcome[J]. Eur Rev Med Pharmacol Sci,2017,21(8):1932-1937.
[3] Chen L,Peng Z,Meng Q,et al. Loss of IκB kinase β promotes myofibroblast transformation and senescence through activation of the ROS-TGFβ autocrine loop[J]. Protein Cell,2016,7(5):338-350.
[4] Kleniewska P,Piechota-Polanczyk A,Michalski L,et al. Influence of block of NF-κappa B signaling pathway on oxidative stress in the liver homogenates[J]. Oxid Med Cell Longev,2013,2013:308358.
[5] Jin M,Lv P,Chen G,et al. Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-κB p65 signaling pathway[J]. Biochem Biophys Res Commun,2017,486(2):451-457.
[6] Qu X,Jiang M,Sun YB,et al. The Smad3/Smad4/CDK9 complex promotes renal fibrosis in mice with unilateral ureteral obstruction[J]. Kidney Int,2015,88(6):1323-1335.
[7] Samarakoon R,Dobberfuhl AD,Cooley C,et al. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation,p53 and reactive oxygen species[J]. Cell Signal,2013,25(11):2198-2209.
[8] Chung S,Jeong JY,Chang YK,et al. Concomitant inhibition of renin angiotensin system and Toll-like receptor 2 attenuates renal injury in unilateral ureteral obstructed mice[J]. Korean J Intern Med,2016,31(2):323-334.
[9] Vu KT,Zhang F,Hulleman JD. Conditional,genetically encoded,small molecule-regulated inhibition of NF-κB signaling in RPE cells[J]. Invest Ophthalmol Vis Sci, 2017,58(10):4126-4137.
[10] Fang Y,Xie T,Xue N,et al. miR-382 contributes to renal tubulointerstitial fibrosis by downregulating HSPD1[J]. Oxid Med Cell Longev,2017,2017:4708516.
[11] Meng XM,Huang XR,Xiao J,et al. Disruption of Smad4 impairs TGF-β/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro[J]. Kidney Int,2012,81(3):266-279.
[12] Zhou TB,Qin YH,Lei FY,et al. Association of prohibitin-1 and 2 with oxidative stress in rats with renal interstitial fibrosis[J]. Mol Biol Rep,2014,41(5):3033-3343.
[13] Strobl MJ,Freeman D,Patel J,et al. Opposing effects of maternal hypo-and hyperthyroidism on the stability of thalamocortical synapses in the visual cortex of adult offspring[J]. Cereb Cortex,2017,27(5):3015-3027.
[14] Chung S,Jeong JY,Chang YK,et al. Concomitant inhibition of renin angiotensin system and Toll-like receptor 2 attenuates renal injury in unilateral ureteral obstructed mice[J]. Korean J Intern Med,2016,31(2):323-334.
[15] Ribeiro LGR,Silva JF,Ocarino NM,et al. Excess maternal and postnatal thyroxine alters chondrocyte numbers and the composition of the extracellular matrix of growth cartilage in rats[J]. Connect Tissue Res,2018,59(1):73-84.
[16] Springer D,Jiskra J,Limanova Z,et al. Thyroid in pregnancy:from physiology to screening[J]. Crit Rev Clin Lab Sci,2017,54(2):102-116.
[17] Wang Y,Sun XL,Wang CL,et al. Influence of screening and intervention of hyperthyroidism on pregnancy outcome[J]. Eur Rev Med Pharmacol Sci,2017,21(8):1932-1937.