糖尿病周围神经病变(diabetic peripheral neuropathy, DPN)是糖尿病常见慢性并发症之一,起病较为隐匿,但危害性较大,可引起疼痛、感觉丧失、足溃疡、坏疽,甚至截肢等[1-2]。20世纪90年代,分批静脉滴注654-2注射液与川芎嗪注射液,促进侧支循环的建立及坏疽创面愈合,在治疗糖尿病足方面取得了令人瞩目的成就。只是654-2注射液与川芎嗪注射液联合应用治疗DPN相关观察指标的数据较少。本研究拟通过不同作用靶点在甲钴胺及依帕司他治疗基础上联合应用小剂量654-2及川芎嗪,探讨其对DPN患者的疗效及安全性,旨在为今后临床治疗相关疾病提供治疗经验,现报告如下。
1 资料与方法
1.1 一般资料 选择2013—2016年华油总医院内分泌科住院的DPN患者60例,按照随机数字表法分为对照组及治疗组各30例。DPN诊断标准:①符合1999年WHO糖尿病诊断标准的2型糖尿病患者;②在诊断糖尿病时或之后出现的神经病变;③临床症状和体征与DPN的表现相符,表现为感觉异常(如疼痛、麻木、烧灼感、蚁走感)、肌无力、肌萎缩等,膝腱及跟腱反射减弱或消失;④5项检查中有2项或2项以上异常则诊断为DPN(a.温度觉异常;b.尼龙丝检查,足部感觉减退或消失;c.振动觉异常;d.踝反射消失;e.神经传导速度有2项或2项以上减慢)。入选标准:①符合DPN诊断标准者;②年龄18~75岁;③入选前2个月血糖控制稳定,糖化血红蛋白<7.5%,空腹血糖<8 mmol/L;④停用其他治疗DPN药物2周以上者。排除标准:①可能与糖尿病神经病变相混淆的疼痛如颈腰椎病变(神经根压迫、椎管狭窄、颈腰椎退行性变)、脑梗塞、格林—巴利综合征、严重动静脉血管病变(静脉栓塞、淋巴管炎)等,尚需鉴别药物尤其是化疗药物引起的神经毒性作用以及肾功能不全引起的代谢毒物对神经损害;②糖尿病单神经病变;③甲状腺功能异常、肝肾功能异常、造血系统疾病、酒精滥用史、电解质异常;④脑血管意外后遗症期;⑤妊娠或哺乳期妇女、精神病患者;⑥青光眼、严重前列腺肥大及下肢动脉血管闭塞患者。2组一般资料差异无统计学意义(P<0.05),具有可比性,见表1。
本研究经医院伦理委员会批准;患者或家属均知情同意并签署知情同意书。
Table 1 Comparison of the clinical data between two groups
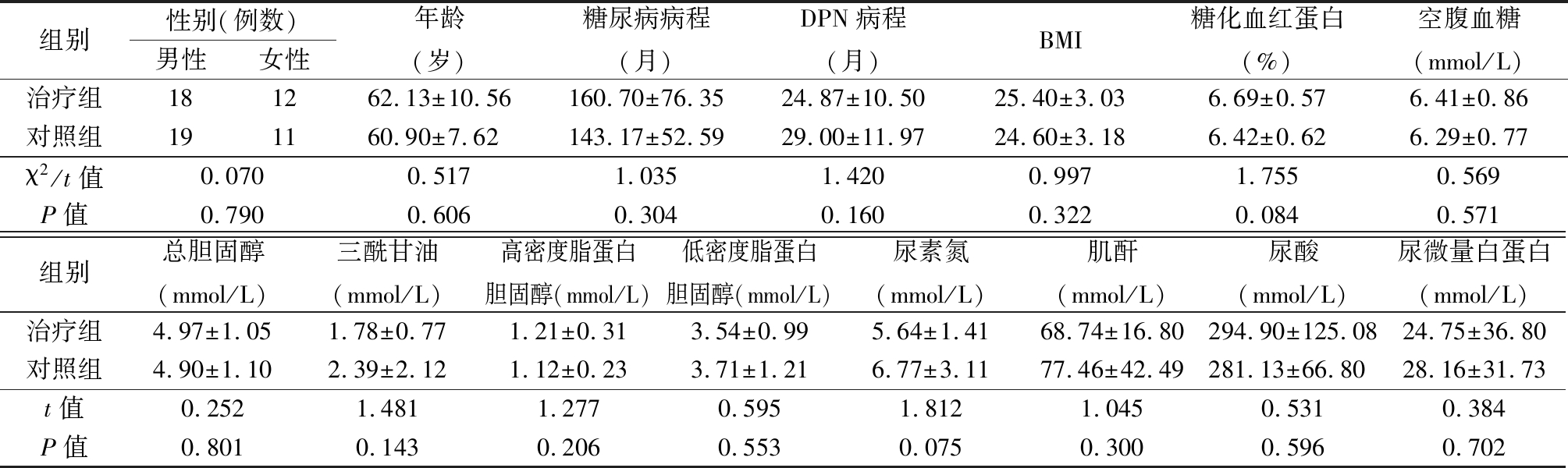
组别性别(例数)男性女性年龄(岁)糖尿病病程(月)DPN病程(月)BMI糖化血红蛋白(%)空腹血糖(mmol/L)治疗组181262.13±10.56160.70±76.3524.87±10.5025.40±3.036.69±0.576.41±0.86对照组191160.90±7.62143.17±52.5929.00±11.9724.60±3.186.42±0.626.29±0.77χ2/t值0.0700.5171.0351.4200.9971.7550.569P值0.7900.6060.3040.1600.3220.0840.571组别总胆固醇(mmol/L)三酰甘油(mmol/L)高密度脂蛋白胆固醇(mmol/L)低密度脂蛋白胆固醇(mmol/L)尿素氮(mmol/L)肌酐(mmol/L)尿酸(mmol/L)尿微量白蛋白(mmol/L)治疗组4.97±1.051.78±0.771.21±0.313.54±0.995.64±1.4168.74±16.80294.90±125.0824.75±36.80对照组4.90±1.102.39±2.121.12±0.233.71±1.216.77±3.1177.46±42.49281.13±66.8028.16±31.73t值0.2521.4811.2770.5951.8121.0450.5310.384P值0.8010.1430.2060.5530.0750.3000.5960.702
1.2 方法 入院后DPN患者均给予糖尿病教育、饮食运动指导、口服降糖药或注射胰岛素等基础治疗控制血糖,并控制血压、血脂等;对照组给予口服甲钴胺片(日本卫材株式会社,国药准字J 20070063)500 μg,3次/d,依帕司他胶囊(扬子江药业集团,50 mg/粒)50 mg,3次/d。治疗组在对照组基础上给予川芎嗪注射液(160 mg)及654-2注射液5 mg加入250 mL生理盐水中静脉滴注,1次/d。疗程均为2周。
1.3 观察指标 比较2组治疗前后超敏C反应蛋白(high sensitive C reaction protein,hsCRP)含量以及多伦多临床评分系统(Toronto Clinical Scoring System,TCSS) 变化。TCSS评分涉及神经症状评分、神经反射评分及感觉功能检查评分三部分,其中6分来自于症状,8分来自于双下肢反射,5分来自于足拇指的感觉,总分0~19分,TCSS诊断DPN的合适截断点为TCSS评分≥6分,见表2。
治疗前后应用肌电图诱发电位仪测定双侧正中神经、腓总神经、胫神经的运动传导速度(motor nerve conduction velocity,MNCV) 和正中神经、腓肠神经感觉传导速度(sensory nerve conduction velocity,SNCV)。
表2 多伦多临床评分系统
Table 2 Toronto Clinical Scoring System (分)
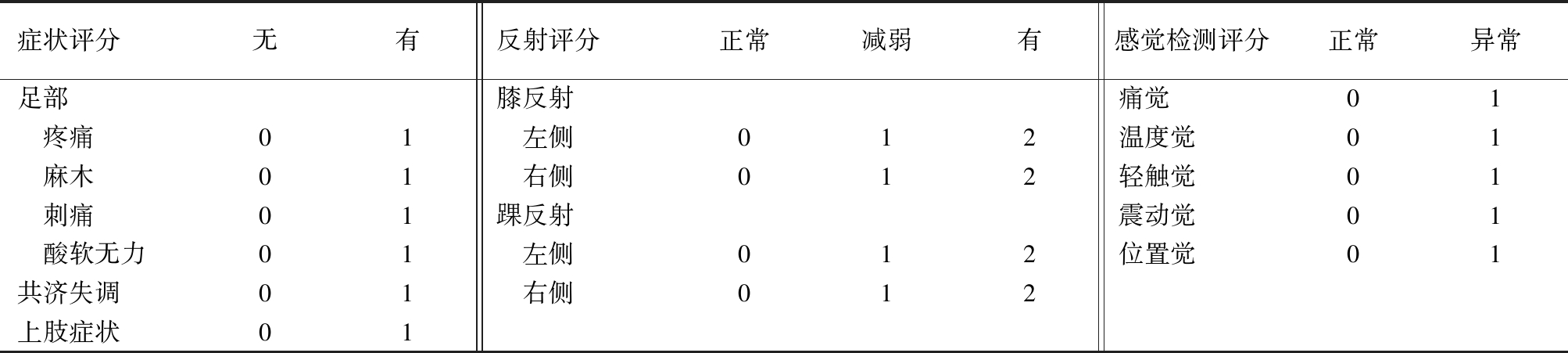
症状评分无有反射评分正常减弱有感觉检测评分正常异常足部膝反射痛觉01 疼痛01 左侧012温度觉01 麻木01 右侧012轻触觉01 刺痛01踝反射震动觉01 酸软无力01 左侧012位置觉01共济失调01 右侧012上肢症状01
1.4 疗效评判标准 神经传导速度评判标准[3]:显效,患者肢体麻木、疼痛、发凉等症状基本或完全消失,神经传导速度较前增加>5 m/s或恢复正常;有效,患者肢体麻木、疼痛、发凉等症状明显改善,神经传导速度较前增加>3 m/s;无效,患者肢体麻木、疼痛、发凉等症状仍有主诉,神经传导速度无改变或较前增加<2.9 m/s。总有效=显效+有效。TCSS评分评判标准[4]:显效,患者肢体麻木、疼痛、乏力等不适明显减轻,自觉症状消失或明显改善,体检神经功能有恢复,TCSS评分下降≥5分;有效,患者肢体麻木、疼痛、乏力等不适轻度减轻,自觉症状好转,TCSS评分下降≥3分;无效,患者症状无改善甚至恶化,TCSS评分下降<3分。总有效=显效+有效。
1.5 统计学方法 应用SPSS 19.0统计软件分析数据。 计量资料比较分别两独立样本的t检验和配对t检验;计数资料比较采用χ2检验;等级资料比较采用秩和检验。 P<0.05为差异有统计学意义。
2 结 果
2.1 2组治疗前后hsCRP含量比较 治疗前,2组hsCRP含量差异无统计学意义(P>0.05);治疗后,2组hsCRP含量均明显低于治疗前,治疗组hsCRP含量明显低于对照组,差异有统计学意义(P<0.05)。见表3。
Table 3 Comparison of hsCRP before and after treatment between two groups
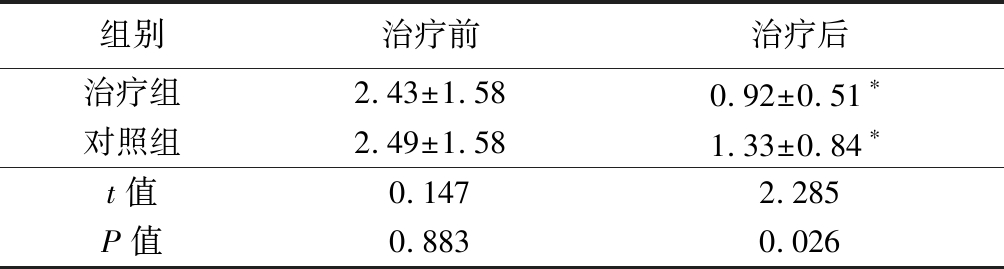
组别治疗前治疗后治疗组2.43±1.580.92±0.51∗对照组2.49±1.581.33±0.84∗t值 0.1472.285P值0.8830.026
*P值<0.05与治疗前比较(配对t检验)
2.2 2组治疗前后神经传导速度比较 治疗前,2组正中神经MNCV、腓总神经MNCV、胫神经MNCV、正中神经SNCV、腓肠神经SNCV差异均无统计学意义(P>0.05)。治疗后,2组正中神经MNCV、腓总神经MNCV、胫神经MNCV、正中神经SNCV、腓肠神经SNCV均明显高于治疗前,且治疗组腓总神经MNCV、胫神经MNCV、正中神经SNCV、腓肠神经SNCV均明显高于对照组,差异有统计学意义(P<0.05);治疗后,2组正中神经MNCV差异无统计学意义(P>0.05)。见表4。
Table 4 Comparison of the nerve conduction velocity before and after treatment between two groups
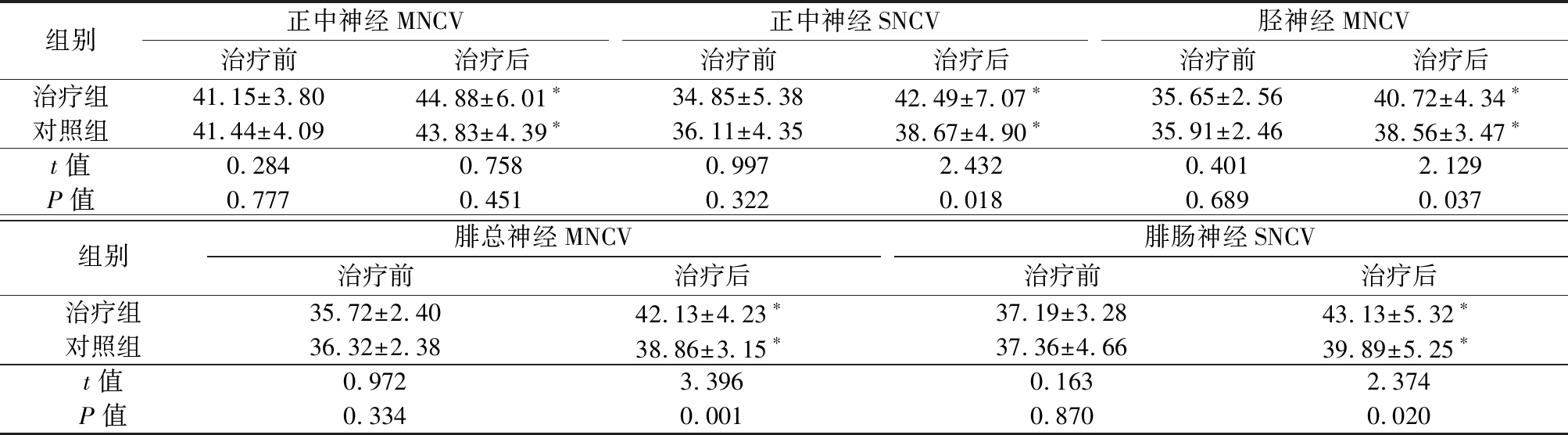
组别正中神经MNCV治疗前治疗后正中神经SNCV治疗前治疗后胫神经MNCV治疗前治疗后治疗组41.15±3.8044.88±6.01∗34.85±5.3842.49±7.07∗35.65±2.5640.72±4.34∗对照组41.44±4.0943.83±4.39∗36.11±4.3538.67±4.90∗35.91±2.4638.56±3.47∗t值0.2840.7580.9972.4320.4012.129P值0.7770.4510.3220.0180.6890.037组别腓总神经MNCV治疗前治疗后腓肠神经SNCV治疗前治疗后治疗组35.72±2.4042.13±4.23∗37.19±3.2843.13±5.32∗对照组36.32±2.3838.86±3.15∗37.36±4.6639.89±5.25∗t值0.9723.3960.163 2.374P值0.3340.0010.8700.020
*P值<0.05与治疗前比较(配对t检验)
2.3 2组临床疗效比较 治疗组临床疗效优于对照组,总有效率高于对照组,差异均有统计学意义(P<0.05),见表5。
表5 2组临床疗效比较
Table 5 Comparison of clinical efficacy between two groups (n=30,例数,%)
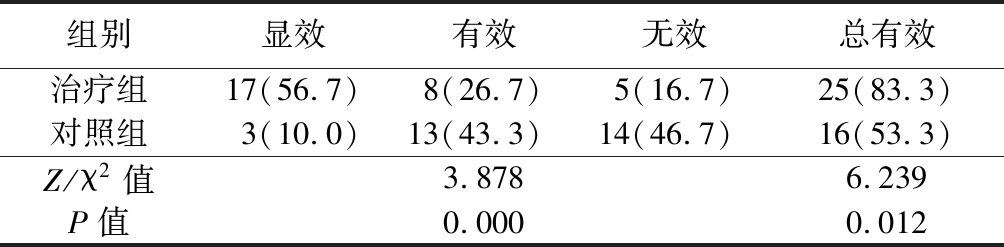
组别显效有效无效总有效治疗组17(56.7)8(26.7)5(16.7)25(83.3)对照组3(10.0)13(43.3)14(46.7)16(53.3)Z/χ2值3.8786.239P值0.0000.012
2.4 2组治疗前后TCSS评分比较 治疗前,2组TCSS评分差异无统计学意义(P>0.05);治疗后,2组TCSS评分均明显低于治疗前,治疗组TCSS评分低于对照组,差异有统计学意义(P<0.05)。见表6。
表6 2组治疗前后TCSS评分比较
Table 6 Comparison of TCSS score before and after treatment between two groups ![]() 分)
分)
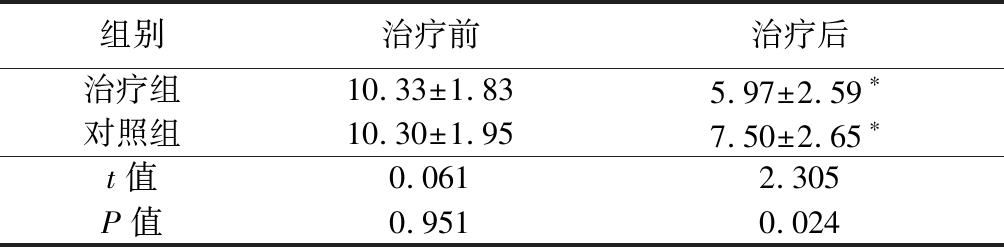
组别治疗前治疗后治疗组10.33±1.835.97±2.59∗对照组10.30±1.957.50±2.65∗t值0.0612.305P值0.9510.024
*P值<0.05与治疗前比较(配对t检验)
2.5 2组TCSS评分疗效比较 治疗组TCSS评分疗效优于对照组,总有效率明显高于对照组,差异有统计学意义(P<0.05),见表7。
表7 2组TCSS评分疗效比较
Table 7 Comparison of clinical efficacy of TCSS score between two groups (n=30,例数,%)
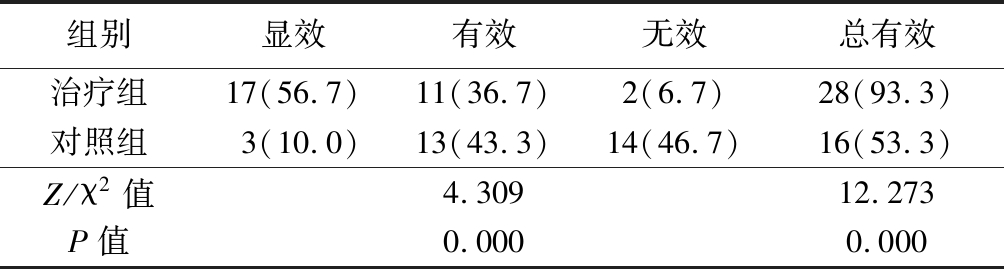
组别显效有效无效总有效治疗组17(56.7)11(36.7)2(6.7)28(93.3)对照组3(10.0)13(43.3)14(46.7)16(53.3)Z/χ2值4.30912.273P值0.0000.000
2.6 2组不良反应比较 在治疗过程中,治疗组出现不同程度口干、面红症状13例,出现排尿不畅6例,停药4 h后上述症状缓解,不良反应发生率为63.3%(19/30);对照组无明显不良反应。所有患者治疗前后心、肝肾功能无异常变化。治疗组不良反应发生率明显高于对照组,差异有统计学意义(χ2=27.804,P=0.000)。
3 讨 论
DPN是糖尿病常见并发症,临床发病率高达52.97%以上[5]。DPN起病隐匿,感觉神经最先受累,也可累及运动神经和自主神经。大多数患者存在对称性肢体麻木、疼痛、感觉异常、蚁走感、灼热感、感觉过敏(呈手套或袜套样感觉)、感觉减退、感觉消失、上腹饱胀、便秘、腹泻、便秘与腹泻交替、大便失禁、排尿费力、尿不尽、心悸、局部多汗、少汗、无汗、手足冰冷等症状,同时伴随着痛觉、温度觉、触压觉、振动觉及腱反射的丧失。虽然DPN临床表现多样,但是主要以持续性疼痛、麻木及感觉减退为主,其中最常见的类型是慢性远端型、对称性、多发性神经病变[5-6]。DPN发病机制尚不十分明确,可能与代谢障碍、血供障碍、神经营养因子缺乏及免疫损伤有关。
甲钴胺是一种内源性辅酶B12,参与脊髓神经元胸腺嘧啶核苷合成,促进叶酸代谢,卵磷脂合成以及神经元髓鞘形成,帮助神经递质和终极板电位诱导恢复正常[7]。依帕司他属于醛糖还原酶抑制剂,通过抑制蛋白激酶 C 信号通路,促进神经内皮细胞中一氧化碳的生成,从而改善患者的神经血管功能[8]。川芎嗪能通过抑制血管生成、纤维化和血栓形成,可促进神经干细胞增殖、抗炎、改善微循环,修复受损的神经纤维组织[9-11]。近年来有研究表明654-2能防止自由基对细胞组织的损伤,减轻炎症反应[12],有效缓解血管痉挛,减少早期血栓形成的风险,改善微循环及缺血组织再灌注[13-14]。
本研究对照组治疗后hsCRP含量、各神经传导速度及TCSS评分等方面均有不同程度提高,但是治疗组上述方面(除正中神经MNCV外)改善程度更加明显,正中神经MNCV改善不明显可能与样本含量小、治疗组患者正中神经运动神经受损害的例数较对照组少有关;在疗效评价方面,以TCSS评分为观察点,治疗组总有效达28例,以神经传导速度为评估指标,治疗组总有效为25例,而对照组在上述两方面治疗的总有效人数无变化。表明在DPN初期一些小神经纤维最先出现脱髓鞘和轴突病理改变,其末梢潜伏期延长和(或)传导速度减慢。不论有无神经病变的临床表现,神经传导均会出现异常,肌电图能较早评估其功能状态[15],但是对于部分无髓鞘及末梢神经病变患者会出现漏诊。如果肌电图与TCSS评分联合评估能更好地反映DPN患者的实际疗效情况,这与一些相关研究吻合[16]。本研究证实小剂量654-2联合川芎嗪能明显提高神经元周围血液灌流量,有效逆转患肢末梢神经细胞缺血缺氧环境,极大提高各神经纤维传导速度,改善周围神经组织的功能状态。
综上所述,DPN应采用针对不同发病机制的多靶点综合治疗。小剂量654-2联合川芎嗪治疗方案治疗DPN是相对安全的,而且经济有效,特别对于基层患者在减少药费及社保费用支出等方面有实际的临床意义。由于本研究样本含量较小,观察时间较短,虽然多数患者的神经症状有改善,但神经传导速度并未达到正常水平。对于DPN患者神经传导速度能否随着治疗的推进而得到逐步改善不得而知,尚有待于进一步观察研究。
[1] Volmer-Thole M,Lobmann R. Neuropathy and diabetic foot syndrome[J]. Int J Mol Sci,2016,17(6):pii:E917.
[2] Amin N,Doupis J. Diabetic foot disease:From the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities[J]. World J Diabetes,2016,7(7):153-164.
[3] 蒋泽.硫辛酸与前列地尔和甲钴胺联合治疗糖尿病周围神经病变[J].实用医药杂志,2012,29(7):595-596.
[4] Han Y,Wang M,Shen J,et al. Differential efficacy of methylcobalamin and alpha-lipoic acid treatment on symptoms of diabetic peripheral neuropathy[J]. Minerva Endocrinol,2018,43(1):11-18.
[5] Weng JP,Bi Y. Epidemiological status of chronic diabetic complications in China[J]. Chin Med J(Engl),2015,128(24):3267-3269.
[6] Doppler K,Reiners K. Diabetic neuropathy:do not only consider distal symmetrical neuropathy[J]. Nervenarzt,2015,86(2):161-166.
[7] Liu J,Sun B,Ban B,et al. Treatment of type 2 diabetic peripheral neuropathy patients of qi-yin deficiency complicated phlegm-dampness blocking collaterals syndrome by internal application of qigui mixture and external application of qigui huoxue lotion:a clinical study[J]. Zhongguo Zhong Xi Yi Jie He Za Zhi,2014,34(9):1053-1058.
[8] Nakamura J,Kamiya H. Diabetic neuropathy[J]. Nihon Rinsho,2015,73(12):2044-2050.
[9] Cai X,Chen Z,Pan X,et al. Inhibition of angiogenesis,fibrosis and thrombosis by tetramethylpyrazine:mechanisms contributing to the SDF-1/CXCR4 axis[J]. PLoS One,2014,9(2):e88176.
[10] Si YC,Li Q,Xie CE,et al. Chinese herbs and their active ingredients for activating xue(blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells[J]. Chin Med,2014,9(1):13.
[11] Xiang F,Wei D,Yang Y,et al.Tissue-engineered nerve graft with tetramethylpyrazine for repair of sciatic nerve defects in rats[J]. Neurosci Lett,2017,638:114-120.
[12] Yuan X,Zheng Y,Chen C,et al. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysisinduced acute kidney injury[J]. Apoptosis,2017,22(12):1524-1531.
[13] Xing K,Fu X,Jiang L,et al. Cardioprotective effect of anisodamine against myocardial ischemia injury and its influence on cardiomyocytes apoptosis[J]. Cell Biochem Biophys,2015,73(3):707-716.
[14] Chen C,Fu X,Li W,et al. Intracoronary administration of anisodamine and nicorandil in individuals undergoing primary percutaneous coronary intervention for acute inferior myocardial infarction:a randomized factorial trial[J]. Exp Ther Med,2015,10(3):1059-1065.
[15] 吕淑娟,邹吉敏,苏盈莹,等.121例糖尿病患者神经电生理分析[J].河北医科大学学报,2016,37(6):640-643.
[16] Pop-Busui R,Boulton AJ,Feldman EL,et al. Diabetic neuropathy:a position statement by the american diabetes association[J]. Diabetes Care,2017,40(1):136-154.