糖尿病肾病是终末期肾病主要原因之一,糖尿病肾病肾小管间质损伤会导致肾功能进一步降低[1]。因此,探讨肾小管间质纤维化原因,寻求有效防治措施具有重要意义。锌α2糖蛋白(zinc-α2-glycoprotein,ZAG)是由脂肪细胞和上皮细胞分泌的糖蛋白,在脂类代谢、细胞循环、肿瘤进展中发挥多种生物学功能[2]。已有研究表明,终末期肾病及血液透析患者血ZAG水平明显升高[3]。ZAG基因缺陷的慢性肾脏病大鼠肾脏ZAG表达降低,肾小管间质损伤加重,给予重组ZAG后肾小管间质纤维化程度减轻,表明ZAG具有抗肾脏纤维化作用[4]。目前,对于ZAG在糖尿病肾病患者肾组织和尿液表达情况研究很少。本研究观察不同程度肾小管间质损伤的糖尿病肾病患者肾组织及尿液ZAG表达水平,并分析其与临床病理指标间的关系,旨在探讨ZAG是否参与了糖尿病肾病肾小管间质损伤过程。
1 资料与方法
1.1 一般资料 选择2016年1月—2017年6月在河北医科大学第二医院肾内科住院根据糖尿病病史、临床检查及肾活检病理确诊的糖尿病肾病患者89例。排除标准:糖尿病合并其他肾脏病、急性肾损伤、狼疮性肾炎、紫癜性肾炎、肿瘤相关性肾脏病、乙型肝炎病毒相关性肾炎等。记录相关临床资料。计算估算的肾小球滤过率(estimated glomerular filtration rate,eGFR)[5]。依据肾病理肾小管间质损伤程度[6]将糖尿病肾病患者分为轻中度组(57例,肾小管间质纤维化面积<50%)和重度组(32例,肾小管间质纤维化面积≥50%)。另选择肾良性肿瘤远离肿瘤的瘤旁正常肾组织10例为对照组。3组性别、年龄差异均无统计学意义(P>0.05),轻中度组与重度组糖尿病病程差异无统计学意义(P>0.05),见表1。
表1 3组相关资料比较
Table 1 Comparison of relevant data among three groups
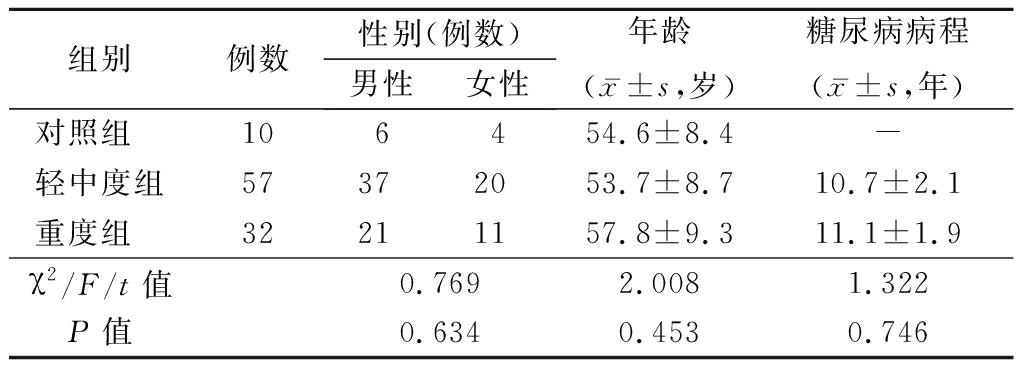
组别 例数性别(例数)男性女性年龄(x-±s,岁)糖尿病病程(x-±s,年)对照组 106454.6±8.4-轻中度组57372053.7±8.710.7±2.1重度组 32211157.8±9.311.1±1.9χ2/F/t值0.7692.0081.322P值0.6340.4530.746
1.2 肾病理肾小管间质损伤程度标准 肾组织2 μm 厚切片,常规脱蜡至水,行PAS染色,光镜观察。计算患者肾小管间质损伤积分:肾间质纤维化和肾小管萎缩,无损伤为0分;损伤面积<25%为1分;损伤面积25%~50%为2分;损伤面积>50%为3分。肾间质炎细胞浸润:无炎细胞浸润为0分;在肾小管间质损伤部位有炎细胞浸润为1分;在无肾小管间质损伤部位也有炎细胞浸润为2分。肾间质血管病变:无内膜增厚为0分;内膜增厚小于中膜厚度为1分;内膜增厚大于中膜厚度为2分。
1.3 肾组织ZAG、转化生长因子β(transforming growth factor-β,TGF-β)表达检测 采用SP 法进行免疫组织化学染色,一抗为兔抗ZAG多克隆抗体、兔抗TGF-β多克隆抗体(购于Proteintech公司),1∶100稀释,二抗为抗兔SP 检测试剂盒,PBS作阴性对照,DAB 显色。应用HPIAS-1000彩色病理图文分析系统采集图像,结果分析应用Image-Pro Plus6.0图像分析软件。ZAG、TGF-β于肾小管上皮细胞和肾间质表达为阳性,取每例标本5个不相同的肾小管间质视野(×200),分析阳性面积和染色深度,计算阳性区域的积分光密度,比较各组均值。
1.4 尿ZAG水平检测 采用酶联免疫吸附测定法检测,留取对照组及糖尿病肾病患者晨尿10 mL,常温下离心5 min(3 000 r/min),弃上清液,将尿沉渣置于-20 ℃冰箱冷冻保存。检测时将留取的尿沉渣常温复温30 min,采用人ZAG 酶联免疫吸附测定试剂盒(购于Raybiotech公司) 检测,微孔板每孔加入100 μL抗ZAG抗体工作液,室温孵育90 min,弃去孔内液体,洗板甩干;分别将系列标准品、阳性对照、尿液标本稀释液100 μL加入孔内, 室温孵育150 min;弃去孔内液体,洗板甩干;链霉亲和素-HRP 100 μL 于室温孵育60 min,洗板拍干后加人显色剂, 室温避光显色10 min, 加人终止液50 μL,以色谱酶标仪于450 nm 波长下读取吸光度值, 根据标准品浓度及OD值计算标准曲线方程, 进一步计算得出尿ZAG表达水平。
1.5 统计学方法 应用SPSS 19.0统计软件分析数据。计量资料比较分别采用两独立样本的t检验、F检验和SNK-q检验;计数资料比较采用χ2检验;相关性采用Spearman相关分析。P<0.05为差异有统计学意义。
2 结 果
2.1 3组临床资料及肾小管间质损伤积分比较 轻中度组和重度组糖化血红蛋白、体重指数、胆固醇、收缩压、舒张压、尿蛋白定量、肾小管间质损伤积分均高于对照组,eGFR、白蛋白、尿ZAG均低于对照组(P<0.05);重度组尿蛋白定量、肾小管间质损伤积分及尿ZAG均高于轻中度组,eGFR低于轻中度组(P<0.05)。轻中度组与重度组糖化血红蛋白、体重指数、胆固醇、收缩压、舒张压、白蛋白差异均无统计学意义(P>0.05)。见表2。
表2 3组临床资料及肾小管间质损伤积分比较
Table 2 Comparison of clinical data and renal pathological scores among three groups![]()
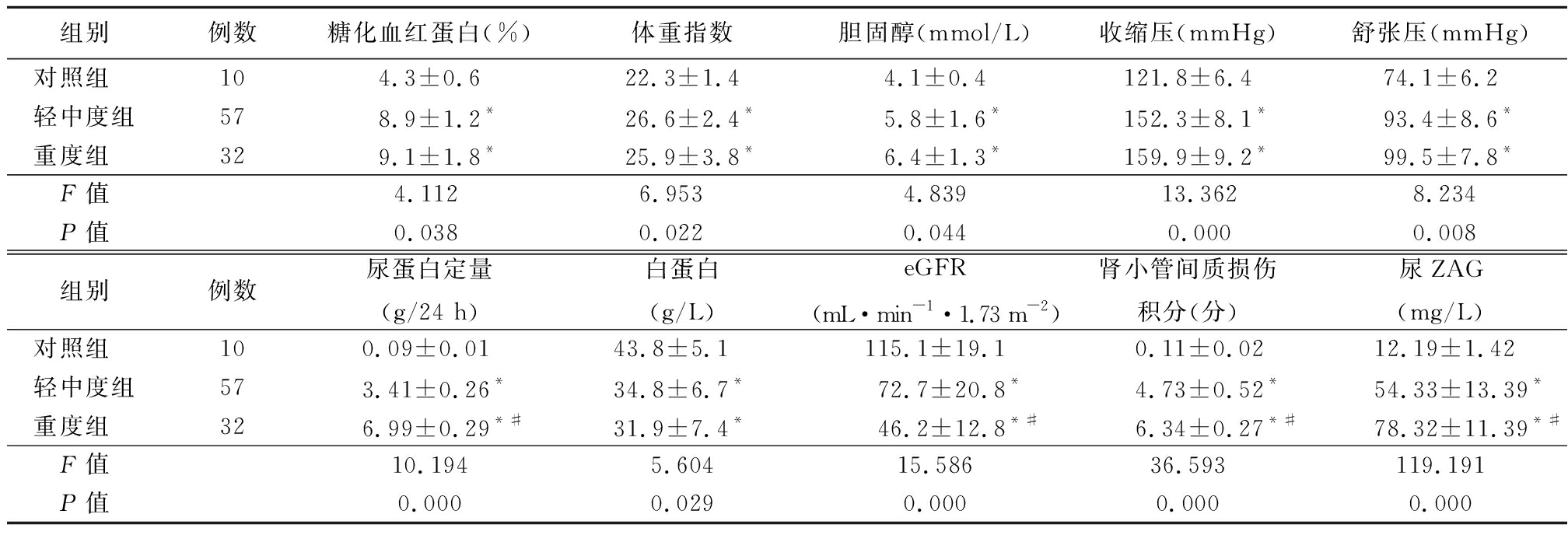
组别例数糖化血红蛋白(%)体重指数胆固醇(mmol/L)收缩压(mmHg)舒张压(mmHg)对照组 104.3±0.622.3±1.44.1±0.4121.8±6.474.1±6.2轻中度组578.9±1.2*26.6±2.4*5.8±1.6*152.3±8.1*93.4±8.6*重度组 329.1±1.8*25.9±3.8*6.4±1.3*159.9±9.2*99.5±7.8*F值4.1126.9534.83913.3628.234P值0.0380.0220.0440.0000.008组别例数尿蛋白定量(g/24 h)白蛋白(g/L)eGFR(mL·min-1·1.73 m-2)肾小管间质损伤积分(分)尿ZAG(mg/L)对照组 100.09±0.0143.8±5.1115.1±19.10.11±0.0212.19±1.42轻中度组573.41±0.26*34.8±6.7*72.7±20.8*4.73±0.52*54.33±13.39*重度组 326.99±0.29*#31.9±7.4*46.2±12.8*#6.34±0.27*#78.32±11.39*#F值10.1945.60415.58636.593119.191P值0.0000.0290.0000.0000.000
*P值<0.05与对照组比较 #P值<0.05与轻中度组比较(SNK-q检验);1 mmHg=0.133 kPa
2.2 糖尿病肾病肾组织PAS病理结果 轻中度组肾病理表现为肾小球基底膜轻度增厚,系膜区增宽,轻度局灶肾小管上皮细胞颗粒变性,小片状肾间质纤维化。重度组肾病理表现为肾小球基底膜重度弥漫增厚,系膜基质明显增多,可见典型的K-W结节或球性硬化,弥漫性肾小管萎缩及肾间质纤维化。
2.3 ZAG在肾组织的表达 ZAG在对照组肾小球少量表达,在肾小管上皮细胞及肾间质中明显表达。ZAG在糖尿病肾病患者肾小球几乎不表达,轻中度组肾小管间质表达较对照组降低,重度组表达较轻中度组明显降低。
2.4 TGF-β在肾组织的表达 TGF-β 在对照组肾小管上皮细胞极少量表达,在糖尿病肾病轻中度组肾小管间质表达增强,糖尿病肾病重度组表达进一步增强。
2.5 ZAG表达与临床指标的相关性分析 糖尿病肾病肾组织ZAG表达水平与eGFR呈正相关,与尿蛋白定量、肾小管间质损伤积分及TGF-β表达均呈负相关(P<0.05);尿液ZAG水平与eGFR呈负相关,与肾小管间质损伤积分呈正相关(P<0.05)。见表3。
表3 糖尿病肾病肾组织ZAG及尿液ZAG与临床指标的相关性
Table 3 Correlation of kidney ZAG and urine ZAG with clinical indicators in diabetic nephropathy
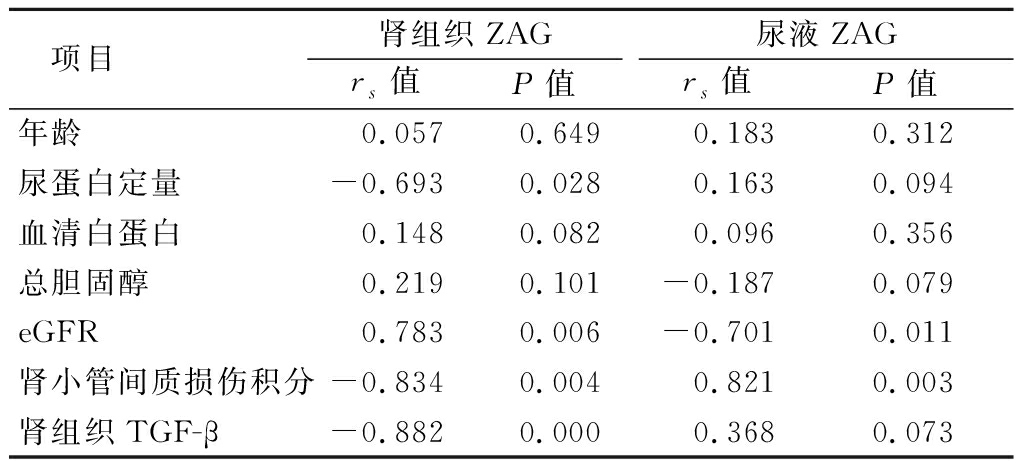
项目肾组织ZAGrs值P值尿液ZAGrs值P值年龄0.0570.6490.1830.312尿蛋白定量-0.6930.0280.1630.094血清白蛋白0.1480.0820.0960.356总胆固醇0.2190.101-0.1870.079eGFR0.7830.006-0.7010.011肾小管间质损伤积分-0.8340.0040.8210.003肾组织TGF-β-0.8820.0000.3680.073
3 讨 论
糖尿病肾病在中国已成为慢性肾脏病首位原因,严重危害人类生活与健康[7]。肾小管间质纤维化在糖尿病肾病进展中起到至关重要的作用[8]。肾小管上皮细胞转分化是肾小管间质纤维化发病机制之一,转分化的肾小管上皮细胞丢失上皮细胞标志蛋白,而获得间充质细胞特性如波形蛋白、平滑肌肌动蛋白等升高,而转变为成纤维细胞纤维细胞,推动肾间质纤维化进展[9]。
脂肪细胞因子ZAG是脂肪细胞和上皮细胞分泌的一种细胞因子,属于Ⅰ类主要组织相容性复合物家族,其基因位于染色体7q22.1,由4个外显子和3个内含子组成。ZAG具有多种生物学功能,能够动员脂肪,促进脂类分解,明显降低体重,使肥胖患者血浆ZAG水平降低。ZAG有潜在抗肥胖、减轻糖尿病胰岛素抵抗的作用[10]。同时也是乳腺癌、前列腺癌等肿瘤的生物学指标之一[11]。ZAG可抑制上皮细胞向间充质转分化,降低肿瘤侵袭性[12]。
正常情况下,ZAG可自由通过肾小球滤过屏障,肾小管上皮细胞重吸收和降解。当肾功能受损时,ZAG清除减少,血浆水平升高。慢性肾脏病、血液透析患者均发现血清ZAG水平增高,有研究认为尿毒症患者血浆ZAG在清除减少情况下,毒素还会刺激脂肪细胞产生过多的ZAG[13]。血清ZAG是血透析患者全因死亡、心血管事件的独立预测因子[14]。在糖尿病肾病患者中,循环血液中ZAG的水平显著升高。 ZAG水平与肌酐、eGFR和尿素有显著的相关性[15]。Elhefnawy等[16]发现糖尿病肾病患者血、尿ZAG明显增高,可以作为此类患者肾损伤的生物学标志物。而对于糖尿病肾病患者肾组织表达研究较少。本研究结果显示,糖尿病肾病组肾组织ZAG表达降低,肾脏纤维化越重,ZAG表达降低越明显;且与尿蛋白、肾小管间质损伤积分呈负相关,与eGFR呈正相关;糖尿病肾病患者尿ZAG水平明显高于对照组。提示ZAG可以作为糖尿病肾病肾损伤的生物标志物。糖尿病肾病尿液中ZAG水平增高与脂肪细胞或上皮细胞分泌生成增多有关[13]。而ZAG在肾组织的低表达可能与其抑制上皮细胞-间充质细胞转分化的作用有关,已发生纤维化的部位,其表达降低。有报道单侧输尿管梗阻及马兜铃酸肾病大鼠,ZAG表达降低伴随α平滑肌肌动蛋白、波形蛋白等成纤维细胞标志蛋白增高,表现为更重的肾间质纤维化[4]。
TGF-β是最主要的致纤维化因子,可以通过多种途径促进肾脏纤维化发生发展[17]。有研究表明,ZAG可通过抑制TGF-β下游ERK通路表达抑制上皮细胞向间充质细胞转分化过程[18]。本研究结果显示,糖尿病肾病肾小管间质TGF-β表达增高,ZAG与TGF-β表达呈负相关。而对于ZAG在肾脏损伤修复过程中是否也通过该途径起作用还需要更进一步的研究证实。
综上所述,糖尿病肾病肾小管间质ZAG表达降低,尿液ZAG水平升高,且随着肾小管间质损伤程度增强而变化更明显。ZAG是糖尿病肾病肾损伤程度的生物学标志物,可能同时具有潜在抗肾脏纤维化作用。今后应进一步研究ZAG抑制肾小管上皮细胞转分化的细胞分子学机制。
[1] Mise K,Hoshino J,Ueno T,et al. Prognostic value of tubulointerstitial lesions,urinary N-Acetyl-β-d-glucosaminidase,and urinary β2-microglobulin in patients with type 2 diabetes and biopsy-proven diabetic nephropathy[J]. Clin J Am Soc Nephrol,2016,11(4):593-601.
[2] Wei X,Liu X,Tan C,et al. Expression and function of zinc-α2-glycoprotein[J]. Neurosci Bull,2019,35(3):540-550.
[3] Bouchara A,Yi D,Pastural M,et al. Serum levels of the adipokine zinc-alpha2-glycoprotein(ZAG) predict mortality in hemodialysis patients[J]. Kidney Int,2018,94(5):983-992.
[4] Sörensen-Zender I,Bhayana S,Susnik N,et al. Zinc-α2-glycoprotein exerts antifibrotic effects in kidney and heart[J]. J Am Soc Nephrol,2015,26(11):2659-2668.
[5] Wood AJ,Churilov L,Perera N,et al. Estimating glomerular filtration rate:performance of the CKD-EPI equation over time in patients with type 2 diabetes[J]. J Diabetes Complications,2016,30(1):49-54.
[6] Li L,Zhang X,Li Z,et al. Renal pathological implications in type 2 diabetes mellitus patients with renal involvement[J]. J Diabetes Complications,2017,31(1):114-121.
[7] Zhang L,Long J,Jiang W,et al. Trends in chronic kidney disease in China[J]. N Engl J Med,2016,375(9):905-906.
[8] Bai X,Hou X,Tian J,et al. CDK5 promotes renal tubulointerstitial fibrosis in diabetic nephropathy via ERK1/2/PPARγ pathway[J]. Oncotarget,2016,7(24):36510-36528.
[9] Han W,Ma Q,Liu Y,et al. Huangkui capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling[J]. Phytomedicine,2019,57:203-214.
[10] Tian M,Liang Z,Liu R,et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: a randomized trial[J]. Eur J Endocrinol,2016,174(2):147-155.
[11] Zhang AY,Grogan JS,Mahon KL,et al. A prospective multicentre phase III validation study of AZGP1 as a biomarker in localized prostate cancer[J]. Ann Oncol,2017,28(8):1903-1909.
[12] Tian H,Ge C,Zhao F,et al. Downregulation of AZGP1 by Ikaros and histone deacetylase promotes tumor progression through the PTEN/Akt and CD44s pathways in hepatocellular carcinoma[J]. Carcinogenesis,2017,38(2):207-217.
[13] Pelletier CC,Koppe L,Croze ML,et al. White adipose tissue overproduces the lipid-mobilizing factor zinc a2-glycoprotein in chronic kidney disease[J]. Kidney Int,2013,83(5):878-886.
[14] Schmitt R. ZAG-a novel biomarker for cardiovascular risk in ESRD patients?[J]. Kidney Int,2018,94(5):858-860.
[15] Xu L,Yu W,Niu M,et al. Serum ZAG levels were associated with eGFR mILD dECrease in T2DM pATIENTS WITH dIABETIC nEPHropathy[J]. Int J Endocrinol,2017,2017:5372625.
[16] Elhefnawy KA,Emad G,Ismail M,et al. Zinc alpha 2 glycoprotein as an early biomarker of diabetic nephropathy in patients with type 2 diabetes mellitus[J]. J Bras Nefrol,2019[Epub ahead of print].
[17] Liu JH,He L,Zou ZM,et al. A novel inhibitor of homodimerization targeting MyD88 ameliorates renal interstitial fibrosis by counteracting TGF-β1-induced emt in vivo and in vitro[J]. Kidney Blood Press Res,2018,43(5):1677-1687.
[18] Xu MY,Chen R,Yu JX,et al. AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways[J]. Cancer Lett,2016,374(2):241-249.