子宫内膜异位症(endometriosis,EMs)是导致育龄期妇女不孕的重要疾病之一[1]。研究显示接受体外受精-胚胎移植(in vitro fertilization and embryo transfer,IVF-ET)的不孕患者中,EMs患者能获取的卵母细胞数较非EMs患者少,卵母细胞质量也有所下降[1-2],可能与颗粒细胞凋亡水平增强有关[3]。生长激素(growth hormone,GH)在生殖功能中具有改善卵巢反应、改善卵子质量、提高子宫内膜容受性的作用,常用于IVF-ET辅助治疗[4]。动物实验发现,GH可抑制排卵前卵泡颗粒细胞凋亡,促使卵子细胞核质同步成熟,从而使卵子的发育潜能得以提升[5-6]。本研究检测EMs患者血清、卵泡液GH水平和颗粒细胞Bcl-2、Bax蛋白表达水平,并对体外培养的EMs患者颗粒细胞使用GH干预,旨在探讨GH对EMs患者的作用及其对颗粒细胞凋亡的影响。
1 资料与方法
1.1 一般资料 选择2017年7月—2018年7月于河北医科大学第二医院生殖医学科行IVF-ET助孕的EMs不孕患者40例为EMs组,选择同期因单纯输卵管因素不孕进行IVF-ET助孕的患者40例为对照组,2组年龄、不孕年限、窦卵泡数、促性腺激素(gonadotropin,Gn)天数和Gn总量差异均无统计学意义(P>0.05),具有可比性(表1)。EMs组均经腹腔镜检查确诊。纳入标准:①年龄<35岁;②月经周期规律,为25~35 d;③能自发排卵;④基础卵泡刺激素(follicle stimulating hormone,FSH)小于10 U/L;⑤体重指数(body mass index,BMI)为18~25;⑥采用超长方案促排卵。排除标准:①子宫性疾病;②近3个月有激素治疗史、吸烟史,卵巢早衰、高泌乳素血症、甲状腺功能异常及多囊卵巢综合征等内分泌疾病。
表1 2组临床指标比较
Table 1 Comparison of the clinical characteristics between two groups (n=40)
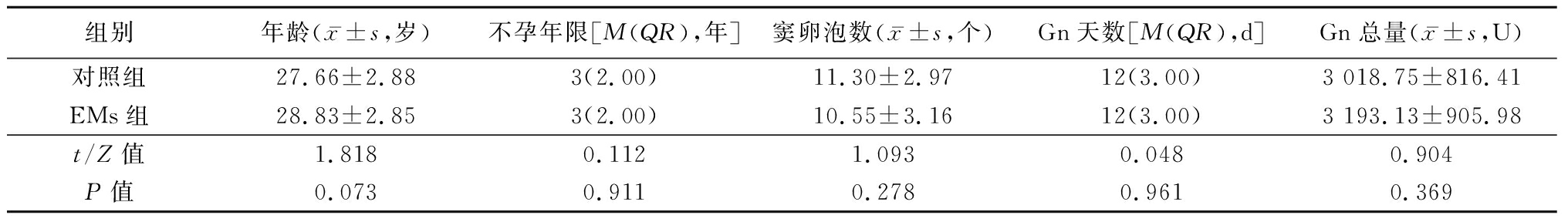
组别年龄(x-±s,岁)不孕年限[M(QR),年]窦卵泡数(x-±s,个)Gn天数[M(QR),d]Gn总量(x-±s,U)对照组27.66±2.883(2.00)11.30±2.9712(3.00)3 018.75±816.41EMs组28.83±2.853(2.00)10.55±3.1612(3.00)3 193.13±905.98t/Z值1.8180.1121.0930.0480.904P值0.0730.9110.2780.9610.369
1.2 主要试剂 10×磷酸盐缓冲液(phosphate buffered saline,PBS)(C10010500BT)、DAB试剂盒(D5905)均为美国Sigma公司产品,免疫组化试剂盒(PK-4001)为美国Vector公司产品,Poly-L-Lysine(P2100)、Bcl-2一抗(sc-492)、Bax一抗(sc-6236)均为美国Santa Cruz公司产品,注射用玻璃酸酶(H31022111)、重组人GH(赛增)(S20050025)为上海第一生化药业有限公司产品,人GH放射免疫分析试剂盒(RP11512)为天津九鼎医学生物工程有限公司产品。
1.3 IVF-ET 采用卵泡期超长方案刺激卵泡发育,阴道超声及血清激素水平监测卵泡发育,适时肌肉注射人绒毛膜促性腺激素(human chorionic gonadotropin,HCG)并取卵。取卵日收集卵泡液及肘静脉血,离心取上清后-80 ℃冻存,按照放射免疫分析试剂盒的操作步骤检测血清和卵泡液GH水平。记录不孕年限、基础窦卵泡数目、Gn用量、使用Gn天数及获卵数。取卵后行常规体外受精,观察受精情况,记录受精卵数、2原核(2 pronucleus,2PN)受精卵数、卵裂数等。取卵后3 d行胚胎移植,将剩余可利用胚胎行玻璃化冷冻保存,记录优质胚胎数、移植胚胎数、冷冻胚胎数等。移植后14 d测血清HCG确定生化妊娠,移植后4周超声检查宫腔内可见孕囊及心管搏动确定临床妊娠。
评价指标的计算:受精率=受精卵数目/获卵总数×100%;2PN受精率=2PN受精卵数/获卵总数×100%;可移植胚胎数=移植胚胎数+冷冻胚胎数;可移植胚胎率=可移植胚胎数/卵裂数×100%;优质胚胎率=优质胚胎数/卵裂数×100%;种植率=种植胚胎数/移植胚胎总数×100%;生化妊娠率=生化妊娠周期数/移植周期数×100%;临床妊娠率=临床妊娠周期数/移植周期数×100%。
1.4 颗粒细胞培养与指标测定
1.4.1 样本制备及细胞培养 卵泡液离心取上清后,吸出沉淀上层黄素化颗粒细胞,玻璃酸酶消化并调整为1×107个/mL的单细胞悬液。将EMs组和对照组颗粒细胞悬液按每孔200 μL接种在置于多孔培养板中的覆有0.05% Poly-L-Lysine涂布的盖玻片上,每孔细胞数均等,置于37 ℃、5% CO2培养箱中预培养。24 h后于倒置显微镜下观察颗粒细胞生长情况。细胞贴壁后,用4%多聚甲醛固定20 min,-20 ℃保存。EMs组换入等量培养液并分为2组,空白组和GH组。GH的终浓度为0.15 μg/mL,每组均设复孔,继续培养24 h,固定后-20 ℃保存,用于免疫细胞化学测定。
1.4.2 免疫细胞化学法测定指标 PBS洗涤细胞玻片后滴加0.3% H2O2去离子水孵育以阻断内源性过氧化物酶。滴加山羊血清室温孵育以阻断非特异性染色。滴加缓冲液稀释的一抗(Bcl-2或Bax),4 ℃过夜。用PBS代替一抗作阴性对照。加入生物素标记的二抗孵育30 min后加入辣根过氧化物酶标记的链酶卵白素孵育。DAB显色后,经苏木素复染,脱水,封片,光学显微镜下观察。阳性染色为胞浆内呈棕黄色颗粒。排除玻片边缘的细胞,随机选取100个细胞,应用HMIAS-2000显微图像分析系统,对细胞进行平均光度值测定。
1.5 统计学方法 应用SPSS 21.0统计软件分析数据。正态分布的计量资料比较采用两独立样本的t检验,非正态分布的计量资料比较采用秩和检验;计数资料比较采用χ2检验。P<0.05为差异有统计学意义。
2 结 果
2.1 2组实验室指标及临床结局比较 2组受精率、2PN受精率、可移植胚胎率和优质胚胎率,以及生化妊娠率、临床妊娠率、种植率差异均无统计学意义(P>0.05),见表2,3。
表2 2组实验室指标比较
Table 2 Comparision of the laboratory characteristics between two groups [n=40,M(QR)]
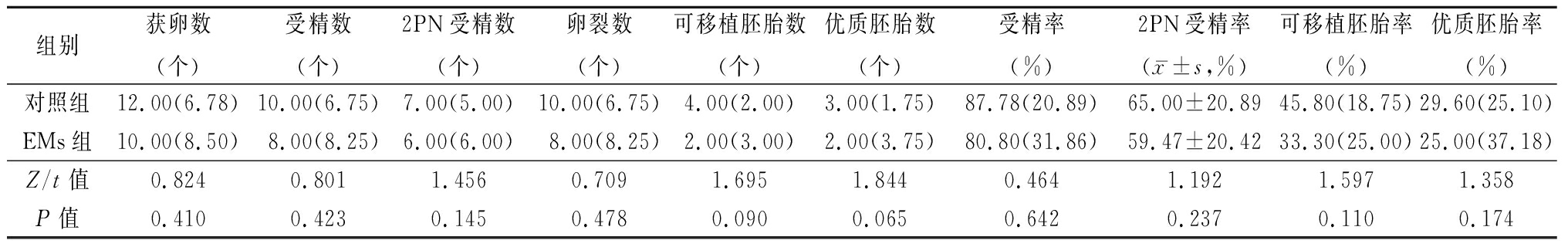
组别获卵数(个)受精数(个)2PN受精数(个)卵裂数(个)可移植胚胎数(个)优质胚胎数(个)受精率(%)2PN受精率(x-±s,%)可移植胚胎率(%)优质胚胎率(%)对照组12.00(6.78)10.00(6.75)7.00(5.00)10.00(6.75)4.00(2.00)3.00(1.75)87.78(20.89)65.00±20.8945.80(18.75)29.60(25.10)EMs组10.00(8.50)8.00(8.25)6.00(6.00)8.00(8.25)2.00(3.00)2.00(3.75)80.80(31.86)59.47±20.4233.30(25.00)25.00(37.18)Z/t值0.8240.8011.4560.7091.6951.8440.4641.1921.5971.358P值0.4100.4230.1450.4780.0900.0650.6420.2370.1100.174
表3 2组临床结局比较
Table 3 Comparision of the clinical outcomes between two groups
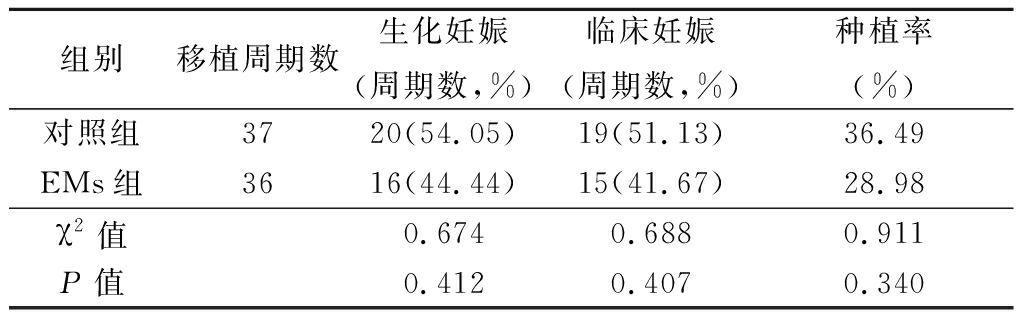
组别移植周期数生化妊娠(周期数,%)临床妊娠(周期数,%)种植率(%)对照组3720(54.05)19(51.13)36.49EMs组3616(44.44)15(41.67)28.98χ2值0.6740.6880.911P值0.4120.4070.340
2.2 2组血清、卵泡液GH水平及颗粒细胞Bcl-2、Bax表达水平比较 EMs组血清、卵泡液GH水平和颗粒细胞Bcl-2表达水平低于对照组,Bax表达水平高于对照组,差异均有统计学意义(P<0.05),见表4。
表4 2组血清及卵泡液GH水平及颗粒细胞Bcl-2、Bax蛋白表达水平比较
Table 4 Comparison of GH level in serum and follicular fluid,expression of Bcl-2 and Bax protein in granulosa cells between two groups (n=40)
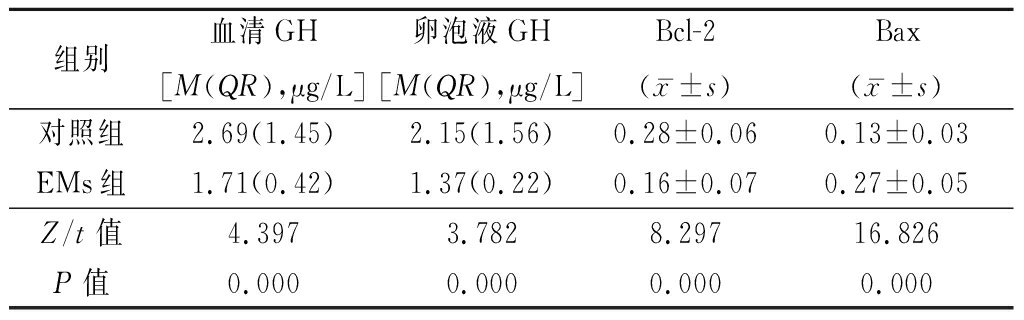
组别血清GH[M(QR),μg/L]卵泡液GH[M(QR),μg/L]Bcl-2(x-±s)Bax(x-±s)对照组2.69(1.45)2.15(1.56)0.28±0.060.13±0.03EMs组1.71(0.42)1.37(0.22)0.16±0.070.27±0.05Z/t值4.3973.7828.29716.826P值0.0000.0000.0000.000
2.3 GH组与空白组EMs颗粒细胞Bcl-2及Bax表达水平比较 GH组较空白组Bcl-2表达水平升高,Bax表达水平下降,差异均有统计学意义(P<0.05),见表5。
表5 GH组与空白组EMs颗粒细胞Bcl-2及Bax蛋白表达比较
Table 5 Comparison of Bcl-2 and Bax protein expression between GH group and blank group![]()
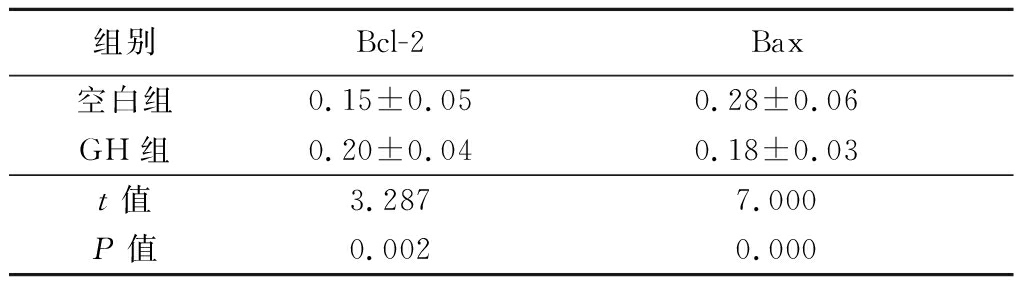
组别Bcl-2Bax空白组0.15±0.050.28±0.06GH组0.20±0.040.18±0.03t值3.2877.000P值0.0020.000
3 讨 论
3.1 颗粒细胞凋亡与EMs 研究显示颗粒细胞与卵母细胞之间存在双向联系,它存在于卵母细胞周围,通过旁分泌通讯调节卵母细胞成熟和卵泡发育[7-8]。颗粒细胞凋亡是介导卵泡发育时期发生卵泡闭锁的因素之一[9]。有研究报道,颗粒细胞凋亡率高,与IVF中空卵泡、取卵数减少、卵母细胞和胚胎质量降低有关[10]。Toya等[11]研究显示EMs不孕患者颗粒细胞的细胞周期异常,凋亡颗粒细胞百分率高于其他研究组(输卵管因素、男性因素、特发性因素),获得的卵母细胞数及成熟卵母细胞数少于男性因素不孕患者。Karuputhula等[12]研究显示,与输卵管因素不孕患者相比,EMs患者颗粒细胞脱氧核糖核酸损伤及凋亡水平增加,并且EMs患者未成熟卵母细胞多于对照组而成熟卵母细胞少于对照组。Goud等[13]研究发现EMs患者的颗粒细胞凋亡水平高于对照组,EMs患者体外成熟至MⅡ期的GV和MⅠ卵母细胞数量显著减少,成熟后的卵母细胞皮质颗粒丢失、透明带硬化、纺锤体和染色体断裂增加。
Bcl-2与Bax是1对抗凋亡基因与促凋亡基因,在卵巢颗粒细胞内表达的比例以及通过二者相互作用所保持的凋亡平衡状态在凋亡过程中起关键作用[14]。Bax使细胞色素C从线粒体漏出,细胞色素C和凋亡蛋白酶激活因子1(apaf-1)与caspase-9结合,从而激活caspase级联,导致细胞死亡。相反Bcl-2通过调节细胞色素C的线粒体释放和apaf-1与caspase-9的相互作用或通过与Bax结合阻断细胞凋亡。本研究中EMs组Bcl-2表达水平低于对照组、Bax表达水平高于对照组(P<0.05);EMs组受精率、2PN受精率、可移植胚胎率和优质胚胎率低于对照组,其差异虽无统计学意义,但表明EMs不孕患者颗粒细胞凋亡水平高于单纯输卵管因素不孕患者,可能影响卵母细胞发育潜能,从而影响卵子质量。
3.2 GH与EMs GH通过下丘脑-垂体轴或直接作用于卵巢影响卵泡功能[15]。研究显示使用GH可显著增加妊娠率和活产率[16],其可作用于颗粒细胞,促进颗粒细胞扩散,激活卵胞浆中的线粒体,降低钙离子浓度,使核质成熟同步,从而提高卵子发育潜能,促进卵裂和胚胎发育[17]。有研究表明,GH并不是通过增加IVF周期获卵数目提高优质胚胎率,而是通过增加颗粒细胞上的GH受体改善卵子质量,提高IVF周期妊娠率及活产率[18]。GH受体存在于人卵母细胞的细胞膜,通过促进GH受体的表达并激活后者的功能活性,提高卵母细胞中线粒体的活力,从而改善卵子质量[19]。GH不仅可以直接作用于性腺靶组织上的受体,也可间接诱导胰岛素样生长因子1(insulin-like growth factor-1,IGF-1)的生成而影响卵泡发育及卵子质量。
本研究中EMs患者血清及卵泡液中GH浓度均低于单纯输卵管因素不孕患者,差异有统计学意义;虽然EMs组与对照组可移植胚胎率及优质胚胎率差异无统计学意义,但EMs患者可移植胚胎率及优质胚胎率均低于单纯输卵管因素不孕患者,且2组生化妊娠率、临床妊娠率、种植率虽然差异无统计学意义,但EMs患者妊娠结局较单纯输卵管因素不孕患者差。表明EMs患者体内GH低水平可能对胚胎质量及妊娠结局产生不良影响,尚需进一步扩大样本量进行研究。
3.3 GH与颗粒细胞凋亡 人颗粒细胞上存在GH受体,GH可能通过作用于颗粒细胞上的GH受体影响颗粒细胞的功能[19]。有研究显示体外培养牛卵巢颗粒细胞加入IGF-1降低了Bax阳性细胞率[20],而体外培养牛窦前卵泡加入IGF-1可增加卵泡发育率[21]。早期研究显示,牛卵巢颗粒细胞体外培养时添加GH不仅导致IGF-1产生增多和凋亡率降低,还导致含有蛋白激酶A(protein kinase A,PKA)催化亚基的细胞比例显著增加,含有PKA调节亚基的细胞比例略有下降[22]。表明GH可能通过调节颗粒细胞IGF-1的自分泌及旁分泌参与卵泡发育而影响卵子质量。IGF-1可通过多种信号转导途径抑制细胞凋亡,其中PI3K/Akt途径与IGF-1对细胞凋亡的保护作用密切相关,IGF-1与IGF-1R结合,激活PI3K/Akt,并通过多种途径保护细胞,如抗凋亡前体基因Bcl-2家族成员BAD基因的磷酸化。BAD基因的磷酸化可阻止其对抗凋亡基因Bcl-xl和Bcl-2的结合,从而减少细胞凋亡。本研究对EMs患者颗粒细胞进行体外培养,GH组与空白组相比,Bcl-2表达水平高、Bax表达水平低,差异有统计学意义。说明一定量的GH可以改善EMs颗粒细胞凋亡状态。
总之,EMs患者血清及卵泡液中GH水平偏低可能导致颗粒细胞凋亡增加,从而影响卵母细胞质量及胚胎的发育潜能,并对IVF-ET的妊娠结局产生不利影响,一定浓度的GH可降低EMs患者颗粒细胞凋亡,改善颗粒细胞凋亡状态,但是否可改善卵母细胞质量而改善IVF-ET妊娠结局仍有待进一步研究。
[1] Gonz lez-Comadran M,Schwarze JE,Zegers-Hochschild F,et al. The impact of endometriosis on the outcome of assisted reproductive technology[J]. Reprod Biol Endocrinol,2017,15(1):8.
lez-Comadran M,Schwarze JE,Zegers-Hochschild F,et al. The impact of endometriosis on the outcome of assisted reproductive technology[J]. Reprod Biol Endocrinol,2017,15(1):8.
[2] Sanchez AM,Vanni VS,Bartiromo L,et al. Is the oocyte quality affected by endometriosis? A review of the literature[J]. J Ovarian Res,2017,10(1):43.
[3] Sanchez AM,Viganò P,Quattrone F,et al. The WNT/beta-catenin signaling pathway and expression of survival promoting genes in luteinized granulosa cells:endometriosis as a paradigm for a dysregulated apoptosis pathway[J]. Fertil Steril,2014,101(6):1688-1696.
[4] Li XL,Wang L,Lv F,et al. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles:a systematic review and meta-analysis[J]. Medicine (Baltimore),2017,96(12):e6443.
[5] Stuczanowska-Gtabowska S,Laszczyńska M,Piotrowska K,et al. The effect of calorie restriction on the presence of apoptotic ovarian cells in normal wild type mice and low-plasma-IGF-1 Laron dwarf mice[J]. J Ovarian Res,2013,6(1):67.
[6] Devesa P,Agasse F,Xapelli S,et al. Growth hormone pathways signaling for cell proliferation and survival in hippocampal neural precursors from postnatal mice[J]. BMC Neurosci,2014,15:100.
[7] Sugiyama M,Sumiya M,Shirasuna K,et al. Addition of granulosa cell mass to the culture medium of oocytes derived from early antral follicles increases oocyte growth,ATP content,and acetylation of H4K12[J].Zygote,2016,24(6):848-856.
[8] Campen KA,Abbott CR,Rispoli LA,et al. Heat stress impairs gap junction communication and cumulus function of bovine oocytes[J]. J Reprod Dev,2018,64(5):385-392.
[9] Hatirnaz S,Ata B,Hatirnaz ES. Oocyte in vitro maturation:a sytematic review[J]. Turk J Obstet Gynecol,2018,15(2):112-125.
[10] Almeida CP,Ferreira MCF,Silveira CO,et al. Clinical correlation of apoptosis in human granulosa cells-A review[J]. Cell Biol Int,2018,42(10):1276-1281.
[11] Toya M,Saito H,Ohta N,et al. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer[J]. Fertil Steril,2000,73(2):344-350.
[12] Karuputhula NB,Chattopadhyay R,Chakravarty B,et al. Oxidative status in granulosa cells of infertile women undergoing IVF[J]. Syst Biol Reprod Med,2013,59(2):91-98.
[13] Goud PT,Goud AP,Joshi N,et al. Dynamics of nitric oxide,altered follicular microenvironment,and oocyte quality in women with endometriosis[J]. Fertil Steril,2014,102(1):151-159.e5.
[14] 徐晓芳,唐利.细胞凋亡在子宫内膜异位症中的相关研究进展[J].中国现代医药杂志,2014,16(1):98-101.
[15] Skowronski MT,Mlotkowska P,Tanski D,et al. Pituitary gonadotropins,prolactin and growth hormone differentially regulate AQP1 expression in the porcine ovarian follicular cells[J]. Int J Mol Sci,2017,19(1).pii:E5.
[16] Yu X,Ruan J,He LP,et al. Efficacy of growth hormone supplementation with gonadotrophins in vitro fertilization for poor ovarian responders:an updated meta-analysis[J]. Int J Clin Exp Med,2015,8(4):4954-4967.
[17] Kuzmina T,Alm H,Denisenko V,et al. Effect of recombinant bovine somatotropin (rbST) on cytoplasmic maturation of bovine oocytes and their developmental competence in vitro[J]. J Reprod Dev,2007,53(2):309-316.
[18] Regan SLP,Knight PG,Yovich JL,et al. Growth hormone during in vitro fertilization in older women modulates the density of receptors in granulosa cells,with improved pregnancy outcomes[J]. Fertil Steril,2018,110(7):1298-1310.
[19] Weall BM,Al-Samerria S,Conceicao J,et al. A direct action for GH in improvement of oocyte quality in poor-responder patients[J]. Reproduction,2015,149(2):147-154.
[20] Sirotkin AV,Ben OA,Tandlmajerov A,et al. cAMP response element-binding protein 1 controls porcine ovarian cell proliferation,apoptosis,and FSH and insulin-like growth factor 1 response[J]. Reprod Fertil Dev,2018,30(8):1145-1153.
A,et al. cAMP response element-binding protein 1 controls porcine ovarian cell proliferation,apoptosis,and FSH and insulin-like growth factor 1 response[J]. Reprod Fertil Dev,2018,30(8):1145-1153.
[21] Jimenez CR,de Azevedo JL,Silveira RG,et al. Effects of growth hormone on in situ culture of bovine preantral follicles aredosedependent[J]. Reprod Domest Anim,2016,51(4):575-584.
[22] Sirotkin AV,Makarevich AV. GH regulates secretory activity and apoptosis in cultured bovine granulosa cells through the activation of the cAMP/protein kinase a system[J]. J Endocrinol,1999,163(2):317-327.