创伤失血性休克为创伤及外科手术期间常见的急性并发症。在休克及其复苏期间极易出现缺血/再灌注胃黏膜应激性损伤,若得不到及时有效治疗,可发展为胃黏膜出血、溃疡、穿孔和上消化道大出血等危及生命的严重并发症[1]。核因子κB(nuclear factor-κB,NF-κB)是一种具有多向性转录调节作用的蛋白因子,对器官缺血/再灌注损伤的发生起重要作用[2-3]。异丙酚为目前临床广泛应用的静脉麻醉药物,除具有催眠、镇静及镇痛作用外,尚可抑制缺血/再灌注心肌和脑组织NF-κB的过度表达,对心脏及脑具有重要的保护作用[3-4]。笔者前期研究发现异丙酚可减轻失血性休克复苏胃黏膜损伤[5],但其对创伤失血性休克复苏胃黏膜损伤(trauma hemorrhagic shock resuscitation-gastric mucosal injury,THSR-GMI)和胃黏膜NF-κB表达的影响及机制目前尚不清楚。本研究通过建立THSR-GMI模型,观察异丙酚对创伤失血性休克复苏胃黏膜NF-κB表达及其炎性反应和细胞凋亡的影响,旨在探讨异丙酚对THSR-GMI的作用及机制。
1 材料与方法
1.1 动物来源及分组 成年雄性新西兰大白兔75只,体重2.5~3.0 kg,由河北省实验动物中心提供。随机分为对照组(S组)、模型组(M组)以及失血前应用异丙酚组(P1组)、复苏前应用异丙酚组(P2组)和复苏后应用异丙酚组(P3组),每组15只。
1.2 实验方法 建立THSR-GMI模型[5]:实验前动物禁食12 h、禁水8 h。3%戊巴比妥钠30 mg/kg静脉注射麻醉成功后,气管切开插管保留自主呼吸,开放耳缘静脉等待输血、输液及给药,右颈外动脉置管接BL-420E+生物机能实验系统(购自成都泰盟科技有限公司)连续监测平均动脉压(mean arterial pressure,MAP)及心率(heart rate,HR),右股动脉插管供放血用。S组执行上述操作,M组、P1组、P2组和P3组上述操作完成后稳定20 min,用骨钳致左股骨中下1/3处粉碎性骨折,右股动脉放血,10 min内使MAP降至35~40 mmHg(1 mmHg=0.133 kPa),并维持60 min后,由股静脉在30 min内回输全部失血及等量生理盐水(复苏)。P1组、P2组和P3组除与M组上述操作相同外,分别于放血前、复苏前10 min及复苏20 min时缓慢静脉注射异丙酚(批号:KW973,意大利Astrazeneca公司)5 mg/kg,后以20 mg·kg-1·h-1持续静脉泵入,S组和M组给予等容量0.9%氯化钠注射液。实验过程中各组均给予0.9%氯化钠注射液10 mL·kg-1·h-1持续静脉泵注,于复苏90 min时股静脉采血1 mL后,以空气注射法杀死动物快速取胃。
1.3 胃黏膜损伤观察及胃黏膜损伤指数(damage index,DI)测定 将所取标本沿胃大弯剪开,冰生理盐水漂洗干净后在冰盘上展平。肉眼观察胃黏膜损伤情况后,在10倍放大镜下参照Guth和Paulsen的方法加以改进,以局限于胃黏膜上皮的点状糜烂、溃疡、出血灶的长度累积计分:正常为0分,损伤≤1 mm计1分,损伤>1~2 mm计2分,损伤>2~3 mm计3分,余类推;损伤宽度>1 mm时加倍计分。每只胃所计总分即为该兔胃黏膜的DI值。
1.4 胃黏膜组织石蜡切片制备及中性粒细胞(polymorphonuclear leukocyte,PMN)计数 取近大弯侧胃全层组织标本0.5 cm×0.5 cm,10%甲醛溶液固定12~24 h,常规梯度乙醇脱水、二甲苯透明、石蜡包埋后连续切片(片厚5 μm)。取石蜡切片,脱蜡至水、苏木精-伊红染色后光镜高倍视野下(×400)观察计数PMN。每张切片随机取10个高倍视野,每只动物随机取3张切片,取其均值即为每只动物的PMN。
1.5 胃黏膜凋亡的检测 按照末端脱氧核苷酰基转移酶介导性dUTP切口末端标记(terminal deoxynucleatidyl transferase-mediated dUTP incision end labeling, TUNEL)试剂盒(购自北京中山生物技术有限公司)说明书操作。取上述石蜡切片脱蜡至水,3%甲醇过氧化氢室温孵育10 min,分别用蒸馏、磷酸盐(phosphate buffered saline,PBS)缓冲洗涤,加入蛋白酶K室温下水解25 min,蒸馏水洗2 min×4次。加入含2%过氧化氢(hydrogen peroxide,H2O2)的PBS,于室温下反应5 min,PBS洗涤5 min×2次。滴加TUNEL反应液(脱氧核糖核苷酸末端转移酶溶液和地高辛标记的核苷酸混合物溶液,在使用前按比例混合),37 ℃孵育1 h。PBS浸洗5 min×3次。滴加过氧化物酶反应物,37 ℃孵育30~40 min,PBS浸洗5 min×3次。3,3-二氨基联苯氨(diaminobenzidine,DAB)、H2O2显色5~10 min,蒸馏水冲洗、苏木精复染、梯度酒精脱水、二甲苯透明、中性树胶封片。光镜下观察胞核中出现棕黄色颗粒为凋亡细胞,计算高倍视野(×400倍)下凋亡细胞数占整个视野细胞总数的百分比,即细胞凋亡指数(apoptoctic index,AI)。每只动物随机取3张切片,每张切片随机分析6个高倍视野,取均值即为每只动物的AI值。
1.6 免疫组织化学染色法测定胃黏膜NF-κB 按照Power Vision二步法免疫组织化学检测系统的要求操作。取上述胃黏膜组织石蜡切片脱蜡至水,加入3% H2O2,用高压锅法修复抗原后,1%小牛血清白蛋白封闭,滴加兔抗兔NF-κB p65多克隆抗体一抗(购于武汉博士德生物技术有限公司),4℃过夜后滴加PV6001复合物(购自北京中山生物技术有限公司),DAB显色、苏木精复染、梯度乙醇脱水、中性树胶封片。阴性对照以PBS缓冲液代替一抗。光镜下胞质或胞核内出现棕黄色颗粒者即为NF-κB阳性细胞。用Leica Qwin图象分析系统(购于德国徕卡公司)检测NF-κB表达灰度值,每只动物随机取3张切片,每张切片随机分析5个高倍(×400倍)视野,取其均值即为每只动物的NF-κB表达灰度值。因阳性表达越强,染色越深,则其灰度值越小,故本研究NF-κB的表达水平用灰度值的倒数表示。
1.7 血清肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)和白细胞介素6(interleukin-6,IL-6)浓度的测定 将所取静脉血,离心取血清置-80 ℃冰箱保存。按照TNF-α和IL-6酶联免疫吸附测定试剂盒(购于美国R&D Systems公司)说明书操作。采用双抗体夹心法,用包被缓冲液将包被抗体(TNF-α和IL-6抗体)稀释至工作浓度,按每微孔100 μL分别包被有TNF-α和IL-6 抗体,4 ℃过夜后洗板,每孔加封闭液,37 ℃湿盒1 h。弃除封闭液后再洗板。加入待测血清,37 ℃孵育30 min,弃去酶标板内液体,洗板3次。加入用辣根过氧化物酶(horseradish peroxidase,HRP)标记的TNF-α抗体或HRP标记的IL-6抗体100μL,37 ℃孵育30 min,弃去酶标板内液体,洗板3次。每孔加入底物溶液100 μL,37 ℃避光孵育30 min,每孔加入终止液50 μL。用标仪450 nm波长下测定每孔的吸光度(OD值),计算待测标本中TNF-α和IL-6的浓度。
1.8 统计学方法 应用SPSS 17.0统计软件分析数据。计量资料比较分别采用F检验及LSD-t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 胃黏膜大体观察 肉眼观察:S组胃黏膜几无损伤性改变;M组全胃充血、水肿,胃黏膜出现较多大面积的条索状或火山口状溃疡,且表面附有出血灶;P1组胃黏膜轻度充血及水肿,无溃疡形成;P3组胃黏膜充血、水肿及散在的小坏死灶和浅表小溃疡形成;P2组胃黏膜损伤情况介于P1组与P3组之间。
2.2 胃黏膜DI值、PMN计数、AI值和NF-κB表达以及血清TNF-α和IL-6浓度变化 与S组比较,M组、P1组、P2组和P3组胃黏膜DI值、PMN计数、AI值、NF-κB表达以及血清TNF-α和IL-6浓度均升高(P<0.05);与M组比较,P1组、P2组和P3组胃黏膜DI值、PMN计数、AI值、NF-κB表达以及血清TNF-α和IL-6浓度均降低(P<0.05);与P3组比较,P1组胃黏膜DI值、PMN计数、AI值、NF-κB表达以及血清TNF-α和IL-6浓度均降低(P<0.05);P1组与P2组相比以及P2组与P3组相比,上述指标差异均无统计学意义(P>0.05)。见表1。
表1 各组兔胃黏膜DI 值、PMN计数、AI值和NF-κB表达以及血清TNF-α、IL-6浓度比较
Table 1 Comparison of Di value,PMN count,AI value,NF-κB expression and serum TNF-α,
IL-6 concentration in gastric mucosa of rabbits in each group![]()
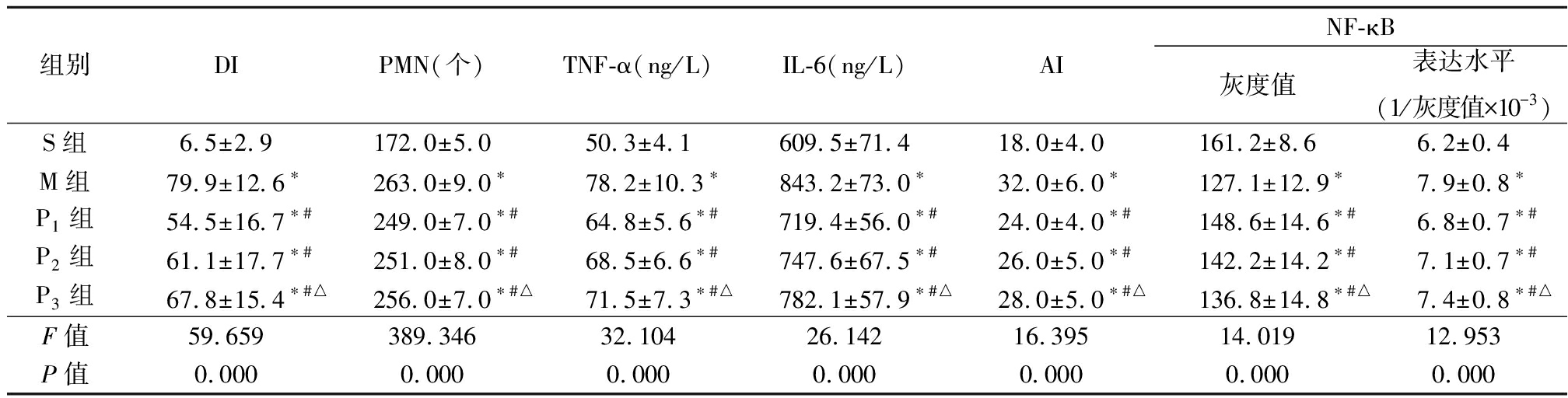
组别DIPMN(个)TNF-α(ng/L)IL-6(ng/L)AINF-κB灰度值表达水平(1/灰度值×10-3)S组6.5±2.9172.0±5.050.3±4.1609.5±71.418.0±4.0161.2±8.66.2±0.4M组79.9±12.6∗263.0±9.0∗78.2±10.3∗843.2±73.0∗32.0±6.0∗127.1±12.9∗7.9±0.8∗P1组54.5±16.7∗#249.0±7.0∗#64.8±5.6∗#719.4±56.0∗#24.0±4.0∗#148.6±14.6∗#6.8±0.7∗#P2组61.1±17.7∗#251.0±8.0∗#68.5±6.6∗#747.6±67.5∗#26.0±5.0∗#142.2±14.2∗#7.1±0.7∗#P3组67.8±15.4∗#△256.0±7.0∗#△71.5±7.3∗#△782.1±57.9∗#△28.0±5.0∗#△136.8±14.8∗#△7.4±0.8∗#△F值59.659389.34632.10426.14216.39514.01912.953P值0.0000.0000.0000.0000.0000.0000.000
*P值<0.05与S组比较 #P值<0.05与M组比较 △P值<0.05与P1组比较(LSD-t检验)
3 讨 论
本研究建立的THSR-GMI模型,肉眼观察胃黏膜损伤严重,且其胃黏膜DI明显升高,表明本实验THSR-GMI模型制备成功。
NF-κB是一种多功能核转录因子,具有广泛的生物学活性[6-7]。研究表明,NF-κB作为炎症反应的标志,不仅调节各种炎性因子的转录表达水平,而且在细胞凋亡中发挥一定的作用[8-9]。缺血/再灌注是一个复杂的过程,炎性反应及细胞凋亡为缺血/再灌注损伤的主要病理生理机制,不仅导致缺血/再灌注损伤的起始和发展,而且在此损伤中发挥着核心作用[10-12]。TNF-α是炎性反应中出现最早、最重要的炎性介质,通过刺激诱导型一氧化氮合酶合成大量的一氧化氮和氧自由基(oxygen free radical,OFR),从而导致组织中细胞膜脂质过氧化损伤,可以用作炎症和组织损伤的一个重要指标[3]。另外,TNF-α可激活PMN在组织中聚集、黏附,并促使IL-1、IL-6及其他继发炎症介质的合成和释放[3,10,13],间接造成或加重胃缺血/再灌注损伤;IL-6主要由活化的中性粒细胞、内皮细胞或血管平滑肌细胞产生的一种重要的促炎细胞因子,是IL-1β下游目标,其过度释放可导致缺血组织的通透性增高,诱导PMN迁移到缺血区,导致或加重组织炎性损伤。因此,IL-6被认为是组织损伤炎症反应最敏感的标志[14],其上调表明炎症的存在[10]。PMN的积累可能参与了细胞凋亡的发病机制,因为PMN释放了多种促炎症细胞因子[10]。而TNF-α和IL-6等参与缺血/再灌注损伤的细胞因子均含有κB位点,受NF-κB的调控。本研究模型组胃黏膜PMN计数、AI值、NF-κB表达以及血清TNF-α和IL-6浓度明显高于对照组,且胃黏膜损伤严重,其DI值也显著高于对照组。提示THSR使胃黏膜细胞NF-κB被激活,诱发炎性反应和细胞凋亡,参与THSR-GMI的发生。其机制可能为:静息状态下,NF-κB以p50和p65二聚体与其抑制蛋白IκB结合为无活性的NF-κB抑制因子(NF-κB-IκB)复合物,存在于胞浆中[6,13,15-16]。而THSR激活炎症反应,其特点为诱导炎性细胞因子释放、PMN浸润及大量的OFR产生[17],OFR及细胞因子通过改变细胞氧化-还原状态,修饰NF-κB激活级联反应中的一个或多个激酶的活性,导致NF-κB从NF-κB-IκB中释放出来,并活化进入胞核,即核易位[13,18],激活的NF-κB与相应的靶基因结合并调节转录、启动细胞间细胞黏附分子1的合成释放,并刺激促炎细胞因子IL-1β、IL-6和TNF-α的活化,诱导炎症反应的下游级联反应,导致组织炎症损害和细胞凋亡反应[19-20],而IL-1、IL-6和TNF-α则可增加NF-κB在生物反馈回路中的活性[3,18,20],放大炎症反应[20]。因此,OFR、NF-κB和炎性细胞因子彼此相互影响,共同参与缺血/再灌注损伤。
短效静脉麻醉药异丙酚,具有抗氧化、抗凋亡和抗炎作用,广泛应用于临床麻醉,发挥神经保护作用,并通过抑制NF-κB的活化而抑制炎症反应,保护大脑和脊髓的缺血/再灌注损伤和改善神经功能[21]。Liu等[13]报道,异丙酚可通过阻止I-κB的磷酸化抑制间歇性缺氧诱导小胶质细胞中NF-κB活性,而且显著降低了促炎性细胞因子TNF-α和IL-6的分泌。另有研究显示,异丙酚通过抑制NF-κB介导的炎症,降低IL-1、IL-6和TNF-α的表达水平和减少细胞凋亡,对内毒素性心肌损伤有保护作用[3]。Wang等[16]报道,异丙酚通过干扰NF-κB信号的激活发挥其对类风湿关节炎成纤维细胞样滑膜细胞的增殖和侵袭的抑制作用。本研究应用异丙酚P1组、P2组和P3组胃黏膜PMN计数、AI值和NF-κB的表达及血清TNF-α和IL-6浓度较M组不同程度明显降低,并且胃黏膜损伤较M组明显减轻,其DI值也显著降低。提示异丙酚亦可抑制NF-κB的表达,减轻炎性反应及细胞凋亡,进而减轻THSR-GMI。其机制可能为:异丙酚其化学成分为2,6-二异丙基甲酚,与内源性抗氧化剂α-生育酚和已知的抗氧化剂丁化羟基甲苯的苯环上均带有一个羟基,具有较强的抗氧化作用[22],可直接与THSR过程中生成的OFR发生反应,生成2,6-二异丙基苯氧基团而使OFR灭活[23-24]。这样既阻断了OFR对NF-κB的活化作用,亦阻断了由NF-κB活化而诱导PMN激活和聚集以及TNF-α、IL-6等炎性细胞因子的产生和细胞凋亡。另外,异丙酚促进膜钙蛋白的表达,对丝裂原活化蛋白激酶系统中p38信号通路的激活产生负调控,从而抑制炎症细胞因子如IL-6的释放[14]。异丙酚尚可通过完全阻断钙、蛋白激酶B和细胞外调节蛋白激酶 1/2信号通路抑制甲酰基肽受体1诱导的人PMN活化[14]。从而对THSR-GMI发挥保护作用。
另外,本研究应用异丙酚3组中,P1组和P2组胃黏膜损伤较P3组轻,并且其DI值、PMN计数、AI值、NF-κB表达水平以及血清TNF-α和IL-6含量均较P3组低,但P1组与P3组的差异尤为显著,此可能与OFR主要在复苏期产生有关。故异丙酚预先用药对减轻THSR-GMI效果均优于其治疗用药,但以缺血前应用效果最佳。
综上所述,异丙酚预先和治疗用药可通过下调NF-κB表达,抑制炎性反应和细胞凋亡,从而减轻THSR-GMI,以缺血前应用效果较佳。
[1] 樊睛伶,高会军,张静瑜,等.止血胶对应激性溃疡的防护作用[J].山西医科大学学报,2019,50(1):20-24.
[2] Huang XW,Pan MD,Du PH,et al. Arginase-2 protects myocardial ischemia-reperfusion injury via NF-κB/TNF-αpathway[J]. Eur Rev Med Pharmacol Sci,2018,22(19):6529-6537.
[3] Yang Z,Cheng F,Yan G,et al. Propofol protects against endotoxin-induced myocardial injury by inhibiting NF-κB-mediated inflammation[J]. Exp Ther Med,2018,15(2):2032-2036.
[4] Zheng Y,Bu J,Yu L,et al. Nobiletin improves propofol-induced neuroprotection via regulating Akt/mTOR and TLR 4/NF-κB signaling in ischemic brain injury in rats[J]. Biomed Pharmacother,2017,91(7):494-503.
[5] 牛竞辉,齐琪,张丽峰,等.异丙酚改善胃黏膜血流量和一氧化氮/内皮素-1平衡减轻兔创伤失血性休克复苏胃黏膜损伤的研究[J].中国急救医学,2017,37(9):830-833.
[6] 王宏,王瑞英,刘志红,等.TGF-β1及NF-κBp65在妊娠期甲亢大鼠子代肾脏中的表达[J].河北医科大学学报,2019,40(3):281-285,291.
[7] 陈亚利,欧阳军,孟晶茜,等.姜黄素对高尿酸血症大鼠肾组织中TGF-β1、NF-κB表达的影响[J].郑州大学学报:医学版,2018,53(3):360-364.
[8] Pan Z,Cui M,Dai G,et al. Protective effect of anthocyanin on neurovascular unit in cerebral ischemia/reperfusion injury in rats[J]. Front Neurosci,2018,12:947.
[9] Liu Y,Chen XD,Yu J,et al. Deletion Of XIAP reduces the severity of acute pancreatitis via regulation of cell death and nuclear factor- κ B activity[J]. Cell Death Dis,2017,8(3):e2685.
[10] Wu J,Yang Y,Xun N,et al. Osthole attenuates myocardial ischemia/reperfusion injury in rats by inhibiting apoptosis and inflammation[J]. Am J Transl Res,2018,10(4):1109-1116.
[11] Gao Y,Song G,Cao YJ,et al. The guizhi gancao decoction attenuates myocardial ischemia-reperfusion injury by suppressing inflammation and cardiomyocyte apoptosis[J]. Evid Based Complement Alternat Med,2019,2019:1947465.
[12] Wang L,Ma H,Xue Y,et al. Berberine inhibits the ischemia-reperfusion injury induced inflammatory response and apoptosis of myocardial cells through the phosphoinositide 3-kinase/RAC-αserine/threonine-protein kinase and nuclear factor-κB signaling pathways[J]. Exp Ther Med,2018 15(2):1225-1232.
[13] Liu S,Sun JY,Ren LP,et al. Propofol attenuates intermittent hypoxia induced up-regulation of proinflammatory cytokines in microglia through inhibiting the activation of NF-κB / p38 MAPK signalling[J]. Folia Neuropathol,2017,55(2):124-131.
[14] Roh GU,Song Y,Park J. Effects of propofol on the inflammatory response during robot-assisted laparoscopic radical prostatectomy:a prospective randomized controlled study[J]. Sci Rep,2019,9(1):5242.
[15] Zhang L,Xu C,Hu W,et al. Anti-inflammatory effects of Lefty-1 in renal tubulointerstitial inflammation via regulation of the NF-κB pathway[J]. Int J Mol Med,2018,41(3):1293-1304.
[16] Wang S,Liang S,Zhao X,et al. Propofol inhibits cell proliferation and invasion in rheumatoid arthritis fibroblast-like synoviocytes via the nuclear factor-κB pathway[J]. Am J Transl Res,2017,9(5):2429-2436.
[17] Wagner N,Dieteren S,Franz N,et al. Ethyl pyruvate ameliorates hepatic injury following blunt chest trauma and hemorrhagic shock by reducing local inflammation,NF-kappaB activation and HMGB1 release[J]. PLoS One,2018,13(2):e0192171.
[18] Zhang J,Tang L,Li GS,et al. The anti-inflammatory effects of curcumin on renal ischemia-reperfusion injury in rats[J]. Ren Fail,2018,40(1):680-686.
[19] Fang R,Zhao NN,Zeng KX,et al. MicroRNA-544 inhibits inflammatory response and cell apoptosis after cerebral ischemia reperfusion by targeting IRAK4[J]. Eur Rev Med Pharmacol Sci,2018,22(17):5605-5613.
[20] Teng Y,Feng C,Liu Y,et al. Anti-inflammatory effect of tranexamic acid against trauma-hemorrhagic shock-induced acute lung injury in rats[J]. Exp Anim,2018,67(3):313-320.
[21] Xie LJ,Huang JX,Yang J,et al. Propofol protects against blood-spinal cord barrier disruption induced by ischemia/reperfusion injury[J]. Neural Regen Res,2017,12(1):125-132.
[22] Zhang Y,Chen Z,Feng N,et al. Protective effect of propofol preconditioning on ischemia-reperfusion injury in human hepatocyte[J]. J Thorac Dis,2017,9(3):702-710.
[23] Li Q,Cui S,Jing G,et al. The role of PI3K/Akt signal pathway in the protective effects of propofol on intestinal and lung injury induced by intestinal ischemia/reperfusion1[J]. Acta Cir Bras,2019,34(1):e20190010000005.
[24] Zhao L,Zhuang J,Wang Y,et al. Propofol ameliorates h9c2 cells apoptosis induced by oxygen glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission[J]. Front Pharmacol,2019,12(10):61.