铁是胎儿正常发育必不可少的微量元素。妊娠期维持铁代谢的稳态至关重要。机体需要储存大约500 mg铁才能满足妊娠期的需求,约40%的女性进入妊娠期会出现铁缺乏的情况。因此,铁缺乏是导致妊娠期贫血最常见的原因之一,对孕期铁代谢的及时监测和评估是防止铁缺乏的必要措施。铁调素是由肝脏分泌的一种富含半胱氨酸的抗菌肽激素,已被确定为铁平衡调节的关键激素[1],可通过抑制十二指肠的铁吸收和巨噬细胞的铁释放负向调控体内铁代谢,在免疫系统、铁运输和铁储存中发挥重要作用[2]。目前有关妊娠期妇女缺铁性贫血中铁调素表达及其调控作用的研究较少。本研究观察不同程度缺铁性贫血孕妇的铁调素表达水平,探讨铁调素与红细胞参数、铁代谢参数、促红细胞生成素(erythropoietin,EPO)及C反应蛋白(C-reactive protein,CRP)之间的关系,进一步阐明铁调素在妊娠期妇女缺铁性贫血中的调节机制,旨在为妊娠期缺铁性贫血的诊疗及预后判断提供重要依据。
1 资料与方法
1.1 一般资料 选择2014年1—12月于我院定期产检的孕妇和体检的健康未孕妇女共80例,分为4组:健康对照组20例,年龄25~34岁,平均(29.50±4.21)岁;孕妇正常组20例,年龄25~34岁,平均(29.30±4.02)岁;孕妇轻度贫血组20例,年龄24~35岁,平均(29.70±5.11)岁;孕妇中度贫血组20例,年龄25~35岁,平均(29.60±4.51)岁。4组年龄差异无统计学意义(P>0.05),具有可比性。
1.2 入选标准 ①贫血组均为妊娠合并缺铁性贫血,孕妇外周血红蛋白(hemoglobin,Hb)<110 g/L,红细胞计数<3.5×1012/L,红细胞压积(hematocrit,Hct)<0.30,红细胞平均体积(mean corpuscular volume,MCV)<80 fl,红细胞平均血红蛋白的含量(mean corpuscular hemogolobin,MCH)<27 pg,红细胞平均血红蛋白浓度(mean corpuscular hemoglobin concentration,MCHC)<320 g/L,血清铁(serum iron,SI)<6.5 μmol/L;②根据Hb浓度,分为轻度贫血组(>90~110 g/L)和中度贫血组(60~90 g/L),由于Hb浓度低于60 g/L的孕妇极其少见,故未设重度贫血组;③孕妇均为单胎妊娠;④无子宫畸形、胎儿先天性发育异常、血红蛋白病;④近3个月未服用铁、叶酸或维生素B12补充剂;⑤无感染或其他炎症性疾病。
1.3 方法 禁食一晚,次日清晨8:00空腹状态下,收集血液标本5~8 mL,其中2 mL加入EDTA-K2抗凝真空管中,与抗凝剂以9∶1的比例充分混匀,用于血常规的检测。另外,剩余全血注射入不含热源和内毒素的促凝管内,3 000 r/min离心10 min,收集上清,-20 ℃保存,用于其他指标的检测,检测前于室温解冻。采用酶联免疫吸附测定法检测铁调素、EPO、血清铁蛋白(serum ferritin,SF)、血清转铁蛋白(serum transferrin, TRF),用酶标仪自带软件采用对数回归计算其浓度;用分光光度法检测SI、总铁结合力(total iron binding force,TIBC);应用 LH750全自动五分类血液分析仪与IMMAGE 800特定蛋白分析系统分别检测血常规和CRP。
1.4 统计学方法 应用SPSS 13.0统计软件分析数据。正态分布的计量资料比较分别采用F检验、SNK-q检验,非正态分布的计量资料以M(QR)表示,比较采用秩和检验。相关性采用Pearson相关分析。P<0.05为有差异有统计学意义。
2 结 果
2.1 4组缺铁性贫血相关参数的比较 与健康对照组比较,孕妇正常组、孕妇轻度贫血组和孕妇中度贫血组Hb、Hct、SI和铁调素均降低,CRP均升高(P<0.05)。与健康对照组比较,孕妇轻度贫血组和孕妇中度贫血组MCV、MCH和MCHC均降低,TIBC均升高(P<0.05)。与健康对照组比较,孕妇中度贫血组EPO升高(P<0.05)。与孕妇正常组比较,孕妇轻度贫血组和孕妇中度贫血组Hb、Hct、MCV、MCH、MCHC、SI和铁调素均降低,TIBC均升高(P<0.05)。与孕妇正常组比较,孕妇中度贫血组EPO和CRP均升高(P<0.05)。与孕妇轻度贫血组比较,孕妇中度贫血组Hb、Hct、MCV、MCH、SI均降低,TIBC和EPO均升高(P<0.05)。4组SF和TRF差异均无统计学意义(P>0.05)。见表1。
表1 4组缺铁性贫血相关参数比较
Table 1 Comparison of related parameters of iron deficiency anemia in four groups![]()
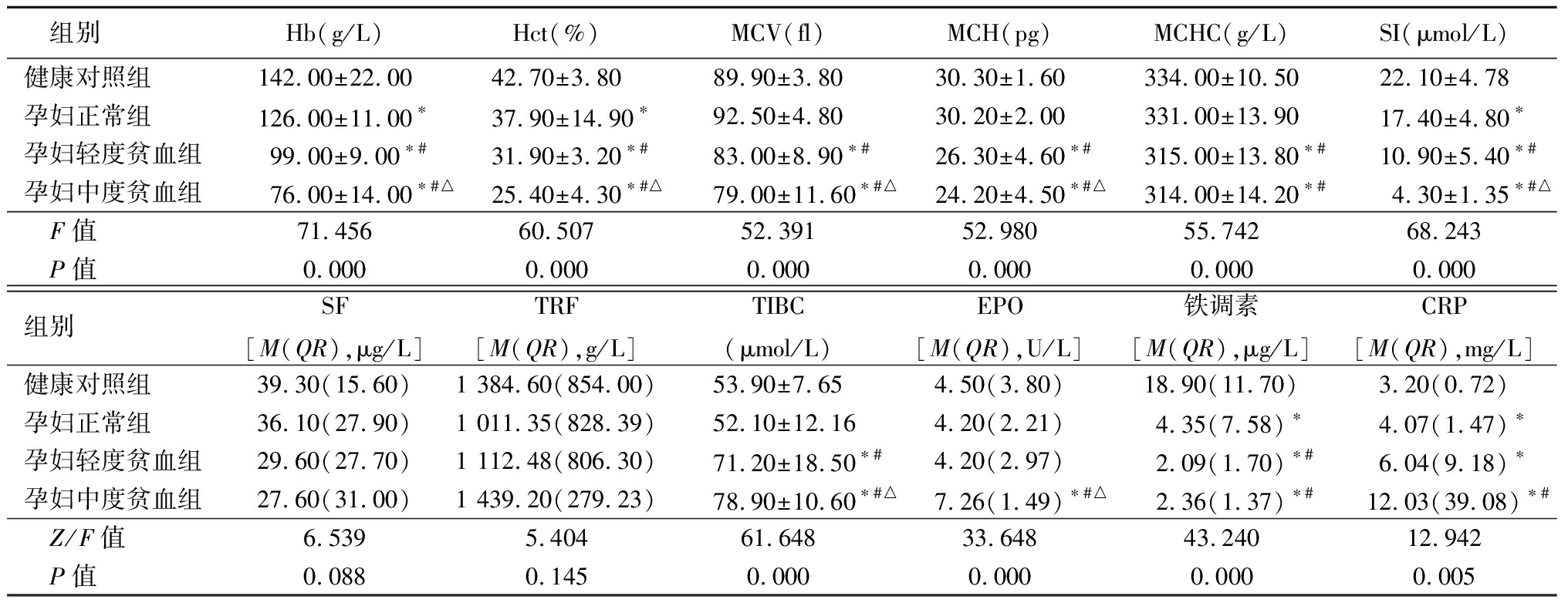
组别Hb(g/L)Hct(%)MCV(fl)MCH(pg)MCHC(g/L)SI(μmol/L)健康对照组142.00±22.0042.70±3.8089.90±3.8030.30±1.60334.00±10.5022.10±4.78孕妇正常组126.00±11.00∗37.90±14.90∗92.50±4.8030.20±2.00331.00±13.9017.40±4.80∗孕妇轻度贫血组99.00±9.00∗#31.90±3.20∗#83.00±8.90∗#26.30±4.60∗#315.00±13.80∗#10.90±5.40∗#孕妇中度贫血组76.00±14.00∗#△25.40±4.30∗#△79.00±11.60∗#△24.20±4.50∗#△314.00±14.20∗#4.30±1.35∗#△ F值71.45660.50752.39152.98055.74268.243 P值0.0000.0000.0000.0000.0000.000组别SF[M(QR),μg/L]TRF[M(QR),g/L]TIBC(μmol/L)EPO[M(QR),U/L]铁调素[M(QR),μg/L]CRP[M(QR),mg/L]健康对照组39.30(15.60)1 384.60(854.00)53.90±7.654.50(3.80)18.90(11.70)3.20(0.72)孕妇正常组36.10(27.90)1 011.35(828.39)52.10±12.164.20(2.21)4.35(7.58)∗4.07(1.47)∗孕妇轻度贫血组29.60(27.70)1 112.48(806.30)71.20±18.50∗#4.20(2.97)2.09(1.70)∗#6.04(9.18)∗孕妇中度贫血组27.60(31.00)1 439.20(279.23)78.90±10.60∗#△7.26(1.49)∗#△2.36(1.37)∗#12.03(39.08)∗# Z/F值6.5395.40461.64833.64843.24012.942 P值0.0880.1450.0000.0000.0000.005
*P值<0.05与健康对照组比较 #P值<0.05与孕妇正常组比较 △P值<0.05与孕妇轻度贫血组比较(SNK-q检验或秩和检验)
2.2 铁调素与各参数的相关性分析 铁调素与Hb、Hct、MCV、MCH、MCHC、SI均呈正相关(r=0.755、0.758、0.604、0.683、0.480、0.716,P<0.05),与TIBC、EPO均呈负相关(r=-0.664、-0.404,P=0.004),与SF、TRF、CRP无相关(r=0.267、-0.216、-0.193,P>0.05)。
3 讨 论
铁是维持机体新陈代谢及内稳态必不可少的微量元素,然而全球范围内仅大约1/5的育龄期妇女有足够的储存铁[3]。由于孕妇及胎儿对铁的需求量增加,机体更容易缺铁,导致缺铁性贫血。目前,评价孕妇铁状态的常用指标有Hct、SF、可溶性转铁蛋白受体等,但妊娠期生理性稀释对其影响较大[4]。近年有研究表明铁调素可作为评价和诊断孕妇缺铁性贫血的有效指标,但妊娠期铁调素调节铁代谢的机制尚未完全阐明。本研究结果显示,妊娠期合并缺铁时铁调素水平明显减低,与Hb、Hct、MCV、MCH、MCHC、SI呈均正相关,与TIBC、EPO呈均负相关。表明妊娠期缺铁性贫血铁调素表达水平的下降可能与EPO的抑制作用有关,这为妊娠期缺铁性贫血的铁代谢调节机制提供了理论依据。
有研究显示,妊娠期间的红细胞参数和铁代谢指标均发生特征性变化,并随贫血程度加重,SI、SF逐渐降低,可溶性转铁蛋白受体逐渐升高[5-6],本研究结果与其一致。铁调素是由肝脏合成的抗菌肽,可通过抑制十二指肠的铁吸收和巨噬细胞的铁释放负向调控机体的铁代谢。Young等[7]研究显示,健康女性铁调素含量与铁的吸收成反比关系。van Santen等[8]研究表明铁调素水平与SI不相关,而与TRF相关。Galesloot等[9]发现血清铁调素与男性和女性的血清SF密切相关。但有关孕妇铁调素表达的研究较少。本研究结果显示,健康孕妇铁调素水平明显低于健康育龄期非妊娠妇女,且贫血孕妇铁调素水平明显低于健康孕妇。
铁调素作为铁代谢调节激素,也参与机体的防御机制,与炎症反应关系密切。有研究表明,CRP是铁调素产生的主要刺激因子,铁调素与CRP水平密切相关,肿瘤患者CRP水平升高,铁调素也相应升高;而肝炎患者铁调素与CRP呈负相关[5-10]。本研究中妊娠期孕妇的CRP虽明显升高,铁调素降低,但CRP是炎性指标,调控因素较多,若探讨CRP与铁调素的相关性尚需进一步实验研究。EPO是由肾脏产生的细胞因子,可直接作用于红系祖细胞刺激造血,促进幼红细胞的成熟,调节红细胞生成。贫血时,骨髓幼红细胞代偿性增生,刺激EPO水平升高,进而骨髓幼红细胞转铁蛋白受体表达升高,增加细胞摄取铁的能力,从而增加Hb合成,同时铁调素的表达降低。铁调素的低表达既能提高十二指肠对铁的吸收,又能促进巨噬细胞、网状内皮系统释放铁。
有研究表明EPO、生长转化因子15和扭转原肠胚形成同系物1均对铁调素 mRNA表达有抑制作用[11]。此外,Finkenstedt等[12]研究显示,妊娠期间血清生长转化因子15、EPO和可溶性铁调素调节蛋白显著增加,铁调素与EPO及可溶性铁调素调节蛋白呈负相关,但与生长转化因子15无明显相关性。给人或小鼠注射EPO可引起剂量依赖性血清铁调素表达量降低,但目前EPO对铁调素的调节机制尚不清楚。本研究结果显示,孕妇中度贫血组EPO水平明显高于其他3组,且EPO与铁调素呈负相关。表明EPO可能抑制铁调素的表达。
总之,随着孕妇缺铁性贫血程度的增加,其血清铁调素水平逐渐下降,同时EPO水平升高。因此,铁调素检测联合红细胞和铁代谢常规参数检测对妊娠期缺铁性贫血的早期发现、诊断、治疗及预后判断有重要的临床应用价值。
[1] Kroot JJ,Tjalsma H,Fleming RE,et al. Hepcidin inhuman iron disorders:diagnostic implications[J]. Clin Chem,2011,57(12):1650-1669.
[2] Ruchala P,Nemeth E. The pathophysiology and pharmacology of hepcidin[J]. Trends Pharmacol Sci,2014,35(3):155-161.
[3] Fisher AL,Nemeth E. Iron homeostasis during pregnancy[J]. Am J Clin Nutr,2017,106(Suppl 6):1567-1574.
[4] He L,Shen C,Zhang Y,et al. Evaluation of serum ferritin and thyroid function in the second trimester of pregnancy[J]. Endocr J,2018,65(1):75-82.
[5] Arezes J,Nemeth E. Hepcidin and iron disorders:new biology and clinical approaches[J]. Int J Lab Hematol,2015,37(Suppl 1):92-98.
[6] Mor G,Cardenas I.The immune system in pregnancy:a uniquecomplexity[J]. Am J Reprod Immunol,2010,63(6):425-433.
[7] Young MF,Glahn RP,Ariza-Nieto M,et al. Serum hepcidin issignificantly associated with iron absorption from food andsupplemental sources in healthy young women[J]. Am J Clin Nutr,2009,89(2):533-538.
[8] van Santen S,Kroot JJ,Zijderveld G,et al. Theironregulatory hormone hepcidin isdecreased in pregnancy:a prospectivelongitudinal study[J]. Clin Chem Lab Med,2013,51(7):1395-1401.
[9] Galesloot TE,Vermeulen SH,Geurts-Moespot AJ,et al. Serum hepcidin:reference rangesand biochemical correlates in the general population[J]. Blood,2011,117(25):e218-225.
[10] Mercadal L,Metzger M,Haymann JP,et al. Therelation of hepcidin to iron disorders,inflammation and hemoglobin in chronic kidneydisease[J]. PLoS One,2014,9(6):e99781.
[11] Hanudel MR,Rappaport M,Chua K,et al. Levels of theerythropoietin-responsive hormone erythroferrone in mice and humans with chronickidney disease[J]. Haematologica,2018,130(4):e141-142.
[12] Finkenstedt A,Widschwendter A,Brasse-Lagnel CG,et al. Hepcidin is correlated to soluble hemojuvelin butnot to increased GDF15 during pregnancy[J]. Blood Cells Mol Dis,2012,48(4):233-237.