三维斑点追踪成像(three-dimensional speckle tracking imaging,3D-STI)技术具有无角度依赖性、实时跟踪斑点运动的立体空间位置等优点,避免几何图形假设所带来的误差,使心脏功能评价的精度提高[1]。本研究旨在探讨3D-STI评估心尖肥厚型心肌病(apical hypertrophic cardiomyopathy,AHCM)患者左心室心肌收缩功能的价值。
1 资 料 与 方 法
1.1 一般资料 选取2016年12月—2018年7月在我院就诊的AHCM患者21例(AHCM组),男性15例,女性6例;年龄35~57岁,平均(49.13±5.61)岁。另选取健康志愿者21例作为对照组,男性15例,女性6例;年龄30~60岁,平均(48.73±6.20)岁。2组性别、年龄差异无统计学意义(P>0.05),具有可比性。图像质量不佳、不能使用斑点追踪成像技术分析者均不能入组。
本研究经医院伦理委员会批准通过;所有受试者均知情同意并签署知情同意书。
1.2 入选标准和排除标准 AHCM患者入选标准:①左心室心尖部厚度≥15 mm;②心尖部厚度与左心室后壁厚度之比≥1.3;③心电图表现为胸前导联倒置对称的巨大T波。排除标准:冠心病、高血压病、尿毒症、主动脉瓣狭窄等可能造成心肌肥厚的疾病,以及影响心脏功能的全身性疾病。对照组入选标准:经过详询病史、体格检查、常规心电图、常规超声心动图检查确定无高血压及影响心脏功能的各种疾病。
1.3 仪器与方法 选用GE vivid E95彩色多普勒超声诊断仪,配备4V-D探头,频率1.5~4.0 MHz。同步连接心电导联后,受试者左侧卧位,在二维模式下测量室间隔及左心室后壁厚度、左心室大小、左心室流出道血流速度,AHCM组同时测量心尖部室壁厚度;在4D模式下,设置为4个心动周期,调整心尖四腔心切面图像,显示标准且清晰后,受试者呼气末屏气时心电触发模式下采集左心室三维动态图像,帧频≥心率×40% 帧/s。
1.4 图像分析 调取存储的图像,点击“measure”键,应用4D Auto LVQ分析软件,选取心尖心内膜及二尖瓣口水平2个点,系统自动勾画心内膜,调整心内膜边界,自动得出左心室舒张末期容积(left ventricular end-diastolic volume,LVEDV)、左心室收缩末期容积(left ventricular end-systolic volume,LVESV)和左心室射血分数(left ventricular ejection fraction,LVEF),调整心内膜及心外膜边界合适后,系统通过程序计算得到纵向应变(longitudinal strain,LS)、圆周应变(circumferential strain,CS)、面积应变(area strain,AS)、径向应变(radial strain,RS)参数及曲线。在分析过程中,出现3个及以上节段被拒绝时,则舍弃该图像。每幅图像均分析3次,取平均值。
1.5 统计学方法 应用SPSS 17.0统计软件分析数据。计量资料比较采用独立样本t检验。P<0.05为差异有统计学意义。
2 结 果
2.1 超声心动图常规检查指标比较 AHCM组LVEDV和SV低于对照组,差异有统计学意义(P<0.05),2组LVESV、LVEF、LVOT-PG差异无统计学意义(P>0.05),见表1。
表1 AHCM组与对照组三维超声心动图常规检查指标比较
Table 1 Comparison of routine echocardiographic parameters between AHCM group and control group ![]()
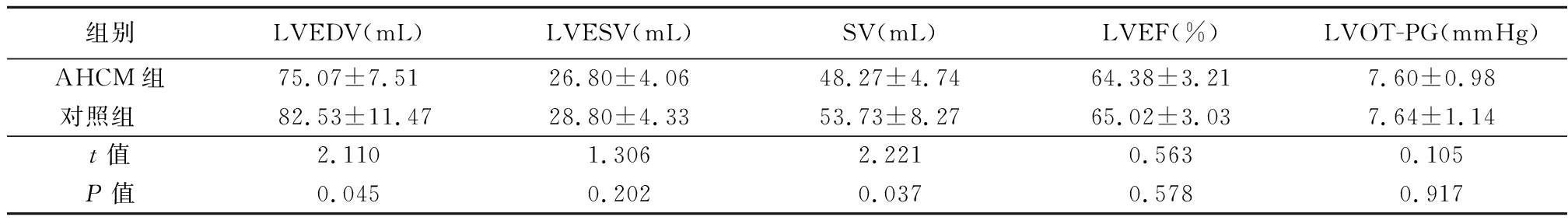
组别LVEDV(mL)LVESV(mL)SV(mL)LVEF(%)LVOT-PG(mmHg)AHCM组75.07±7.5126.80±4.0648.27±4.7464.38±3.217.60±0.98对照组 82.53±11.4728.80±4.3353.73±8.2765.02±3.037.64±1.14t值2.1101.3062.2210.5630.105P值0.0450.2020.0370.5780.917
1 mmHg=0.133 kPa
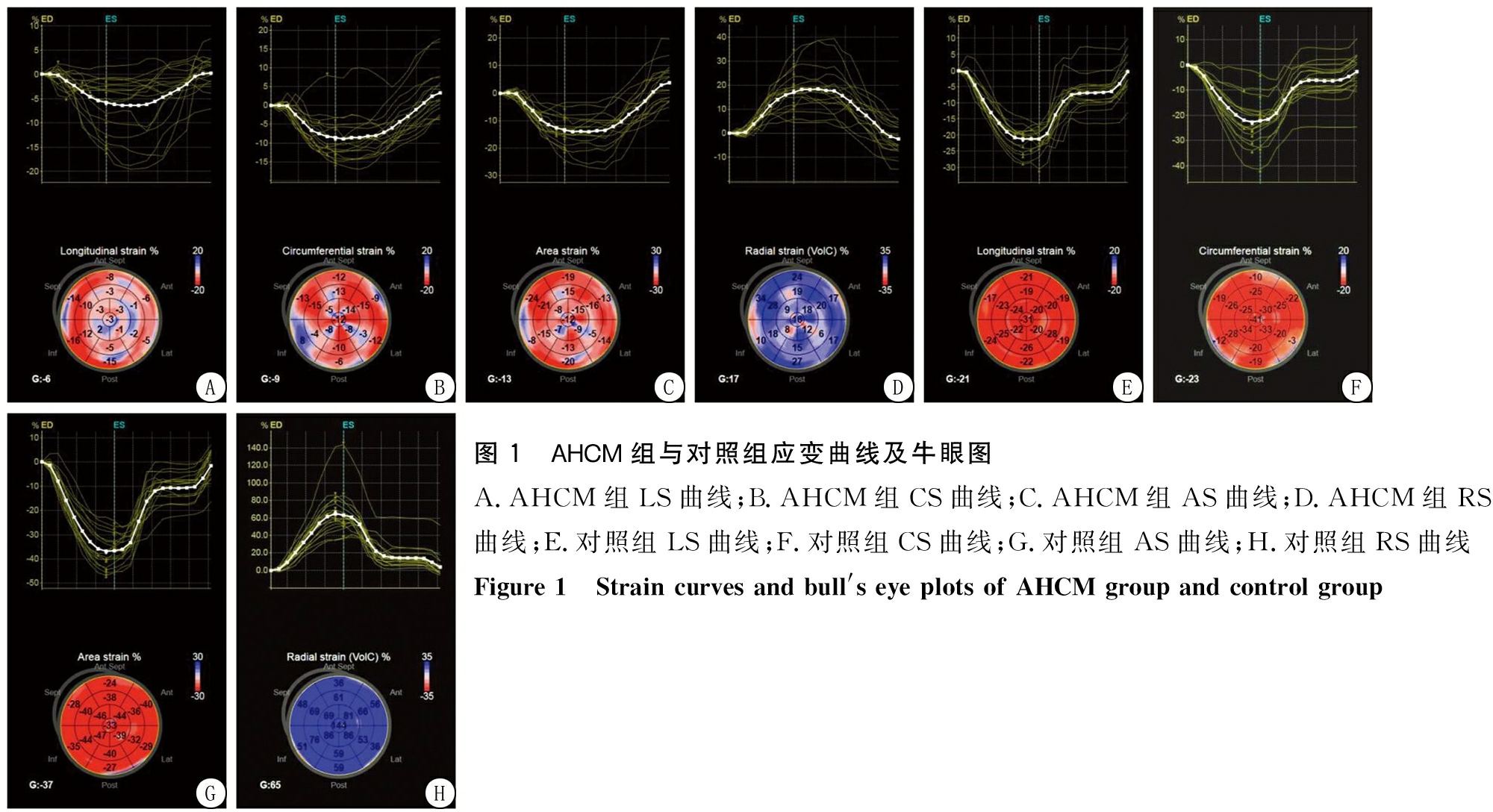
图1 AHCM组与对照组应变曲线及牛眼图A.AHCM组LS曲线;B.AHCM组CS曲线;C.AHCM组AS曲线;D.AHCM组RS曲线;E.对照组LS曲线;F.对照组CS曲线;G.对照组AS曲线;H.对照组RS曲线 Figure 1 Strain curves and bull's eye plots of AHCM group and control group
2.2 左心室整体应变参数比较 AHCM组整体LS、整体CS、整体AS、整体RS值均低于对照组,差异有统计学意义(P<0.01),见表2,图1。
表2 AHCM组与对照组左心室整体应变值比较
Table 2 Comparison of left ventricular global strain between AHCM group and control group ![]()
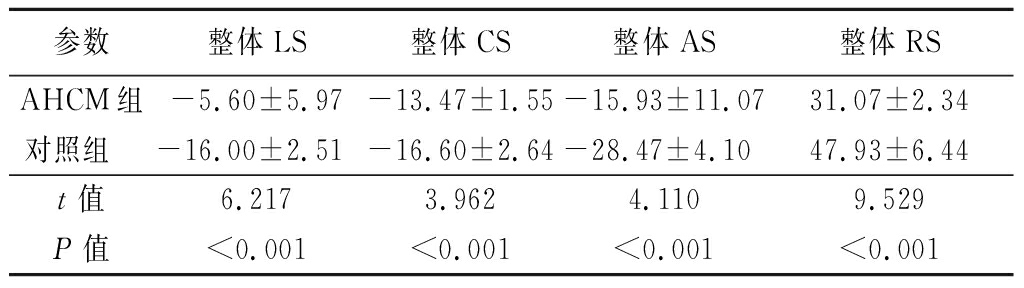
参数整体LS整体CS整体AS整体RSAHCM组-5.60±5.97-13.47±1.55-15.93±11.0731.07±2.34对照组 -16.00±2.51-16.60±2.64-28.47±4.1047.93±6.44t值6.2173.9624.1109.529P值<0.001<0.001<0.001<0.001
2.3 左心室局部应变参数比较 AHCM组二尖瓣水平前壁、侧壁、后壁和后间隔LS低于对照组;乳头肌水平各部位LS、AS、RS值低于对照组,前间隔、前壁、侧壁、后壁CS值低于对照组;心尖水平各部位应变值均低于对照组,差异均有统计学意义(P<0.05或P<0.01)。见表3~6。
表3 AHCM组与对照组左心室各节段LS值比较
Table 3 Comparison of longitudinal strain values of left ventricle in AHCM group and control group ![]()
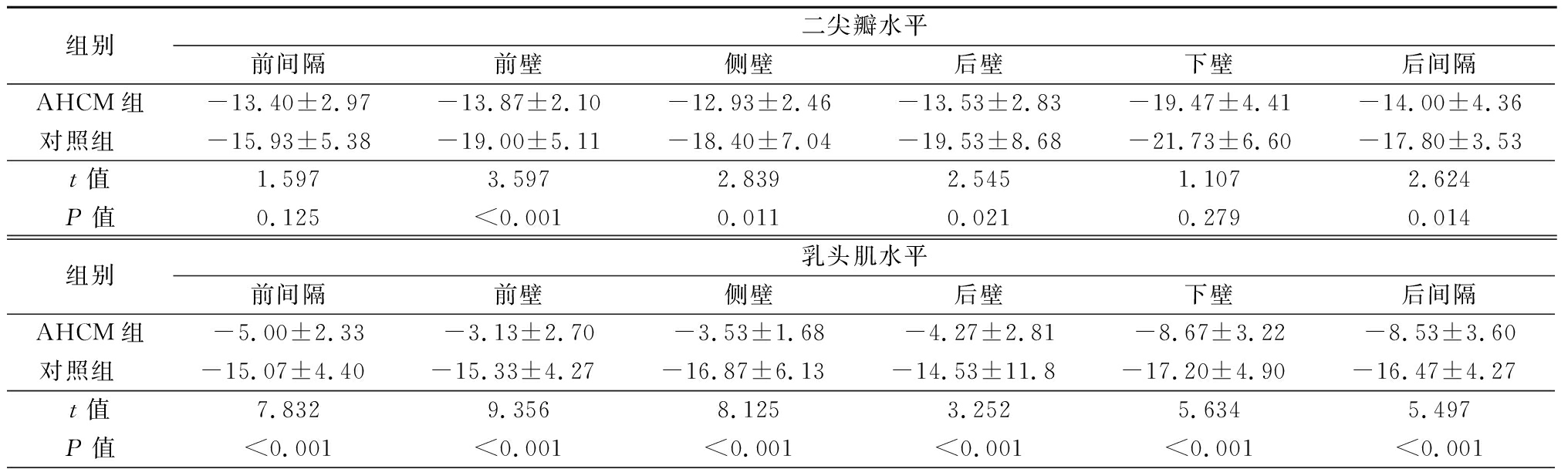
组别二尖瓣水平前间隔前壁侧壁后壁下壁后间隔AHCM组-13.40±2.97-13.87±2.10-12.93±2.46-13.53±2.83-19.47±4.41-14.00±4.36对照组 -15.93±5.38-19.00±5.11-18.40±7.04-19.53±8.68-21.73±6.60-17.80±3.53t值1.5973.5972.8392.5451.1072.624P值0.125<0.0010.0110.0210.2790.014组别乳头肌水平前间隔前壁侧壁后壁下壁后间隔AHCM组-5.00±2.33-3.13±2.70-3.53±1.68-4.27±2.81-8.67±3.22-8.53±3.60对照组 -15.07±4.40-15.33±4.27-16.87±6.13-14.53±11.8-17.20±4.90-16.47±4.27t值7.8329.3568.1253.2525.6345.497P值<0.001<0.001<0.001<0.001<0.001<0.001
表3 (续)
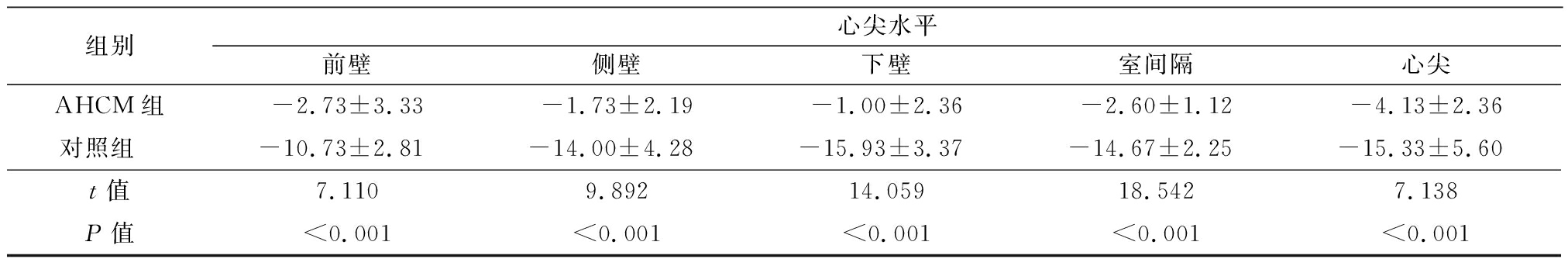
组别心尖水平前壁侧壁下壁室间隔心尖AHCM组-2.73±3.33-1.73±2.19-1.00±2.36-2.60±1.12-4.13±2.36对照组 -10.73±2.81-14.00±4.28-15.93±3.37-14.67±2.25-15.33±5.60t值7.1109.89214.05918.5427.138P值<0.001<0.001<0.001<0.001<0.001
表4 AHCM组与对照组左心室各节段CS值比较
Table 4 Comparison of circumferential strain values of left ventricle in AHCM group and control group ![]()
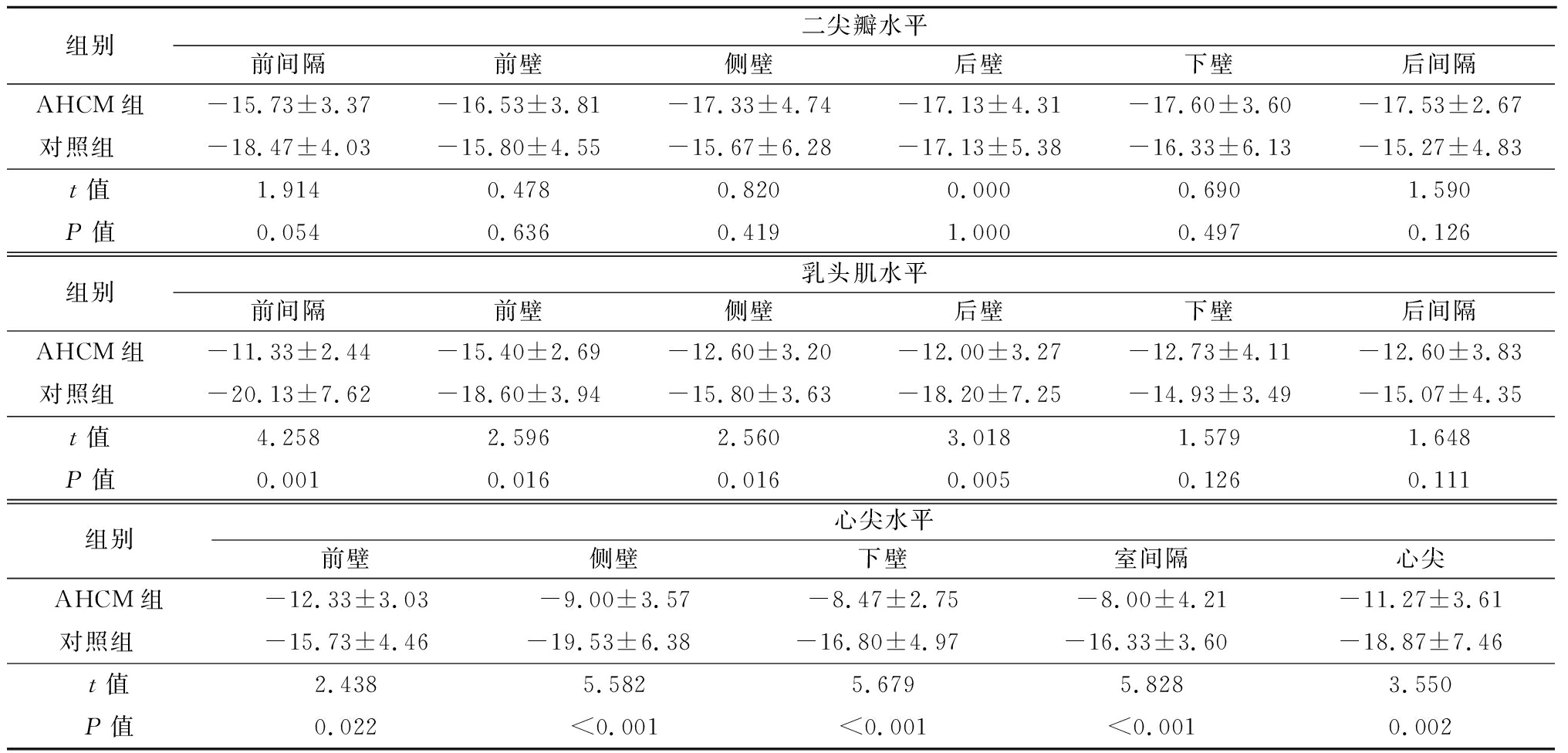
组别二尖瓣水平前间隔前壁侧壁后壁下壁后间隔AHCM组-15.73±3.37-16.53±3.81-17.33±4.74-17.13±4.31-17.60±3.60-17.53±2.67对照组 -18.47±4.03-15.80±4.55-15.67±6.28-17.13±5.38-16.33±6.13-15.27±4.83t值1.9140.4780.8200.0000.6901.590P值0.0540.6360.4191.0000.4970.126组别乳头肌水平前间隔前壁侧壁后壁下壁后间隔AHCM组-11.33±2.44-15.40±2.69-12.60±3.20-12.00±3.27-12.73±4.11-12.60±3.83对照组 -20.13±7.62-18.60±3.94-15.80±3.63-18.20±7.25-14.93±3.49-15.07±4.35t值4.2582.5962.5603.0181.5791.648P值0.0010.0160.0160.0050.1260.111组别心尖水平前壁侧壁下壁室间隔心尖AHCM组-12.33±3.03-9.00±3.57-8.47±2.75-8.00±4.21-11.27±3.61对照组 -15.73±4.46-19.53±6.38-16.80±4.97-16.33±3.60-18.87±7.46t值2.4385.5825.6795.8283.550P值0.022<0.001<0.001<0.0010.002
表5 AHCM组与对照组左心室各节段AS值比较
Table 5 Comparison of area strain values of left ventricle in AHCM group and control group ![]()
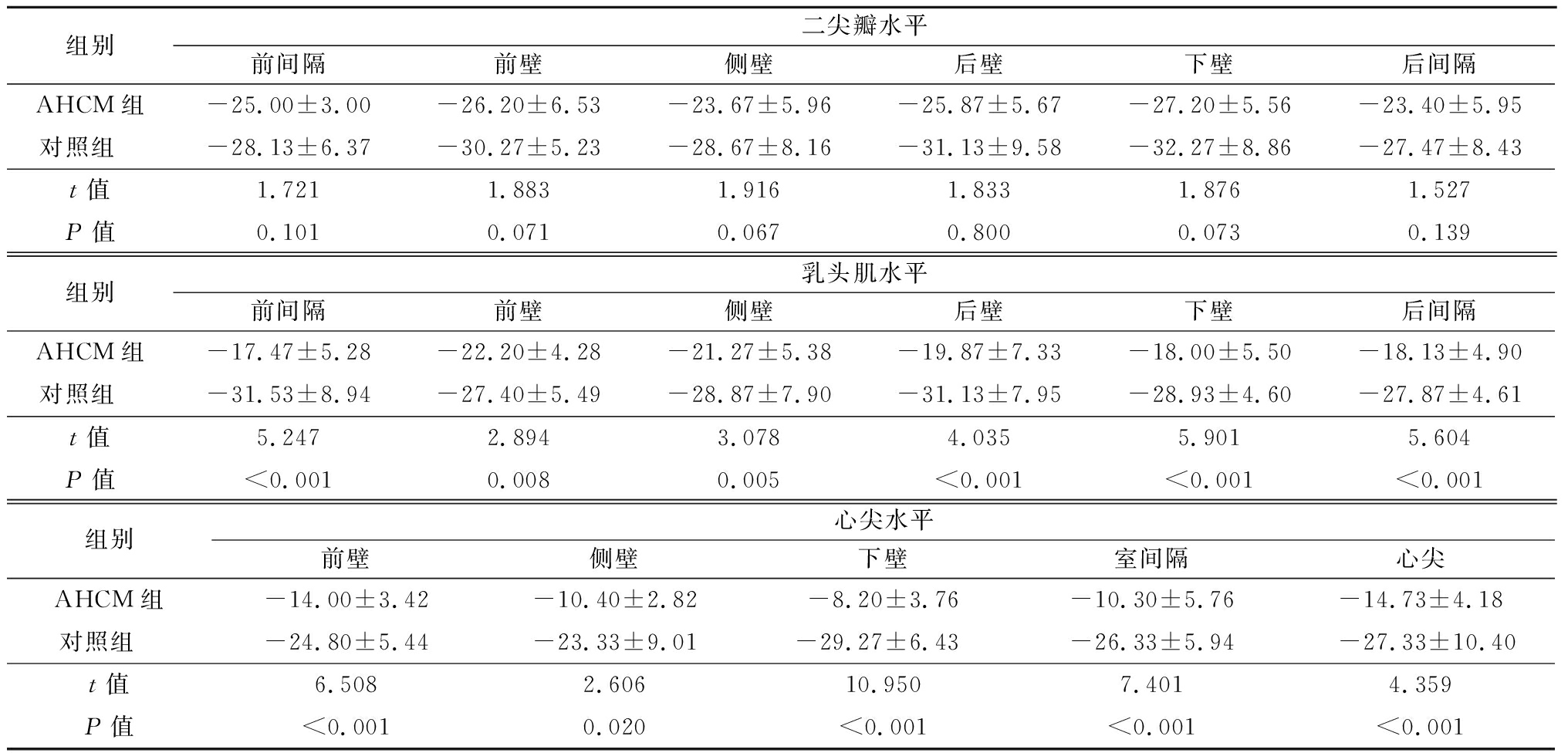
组别二尖瓣水平前间隔前壁侧壁后壁下壁后间隔AHCM组-25.00±3.00-26.20±6.53-23.67±5.96-25.87±5.67-27.20±5.56-23.40±5.95对照组 -28.13±6.37-30.27±5.23-28.67±8.16-31.13±9.58-32.27±8.86-27.47±8.43t值1.7211.8831.9161.8331.8761.527P值0.1010.0710.0670.8000.0730.139组别乳头肌水平前间隔前壁侧壁后壁下壁后间隔AHCM组-17.47±5.28-22.20±4.28-21.27±5.38-19.87±7.33-18.00±5.50-18.13±4.90对照组 -31.53±8.94-27.40±5.49-28.87±7.90-31.13±7.95-28.93±4.60-27.87±4.61t值5.2472.8943.0784.0355.9015.604P值<0.0010.0080.005<0.001<0.001<0.001组别心尖水平前壁侧壁下壁室间隔心尖AHCM组-14.00±3.42-10.40±2.82-8.20±3.76-10.30±5.76-14.73±4.18对照组 -24.80±5.44-23.33±9.01-29.27±6.43-26.33±5.94-27.33±10.40t值6.5082.60610.9507.4014.359P值<0.0010.020<0.001<0.001<0.001
表6 AHCM组与对照组左心室各节段RS值比较
Table 6 Cmparison of radial strain values of left ventricle in AHCM group and control group ![]()
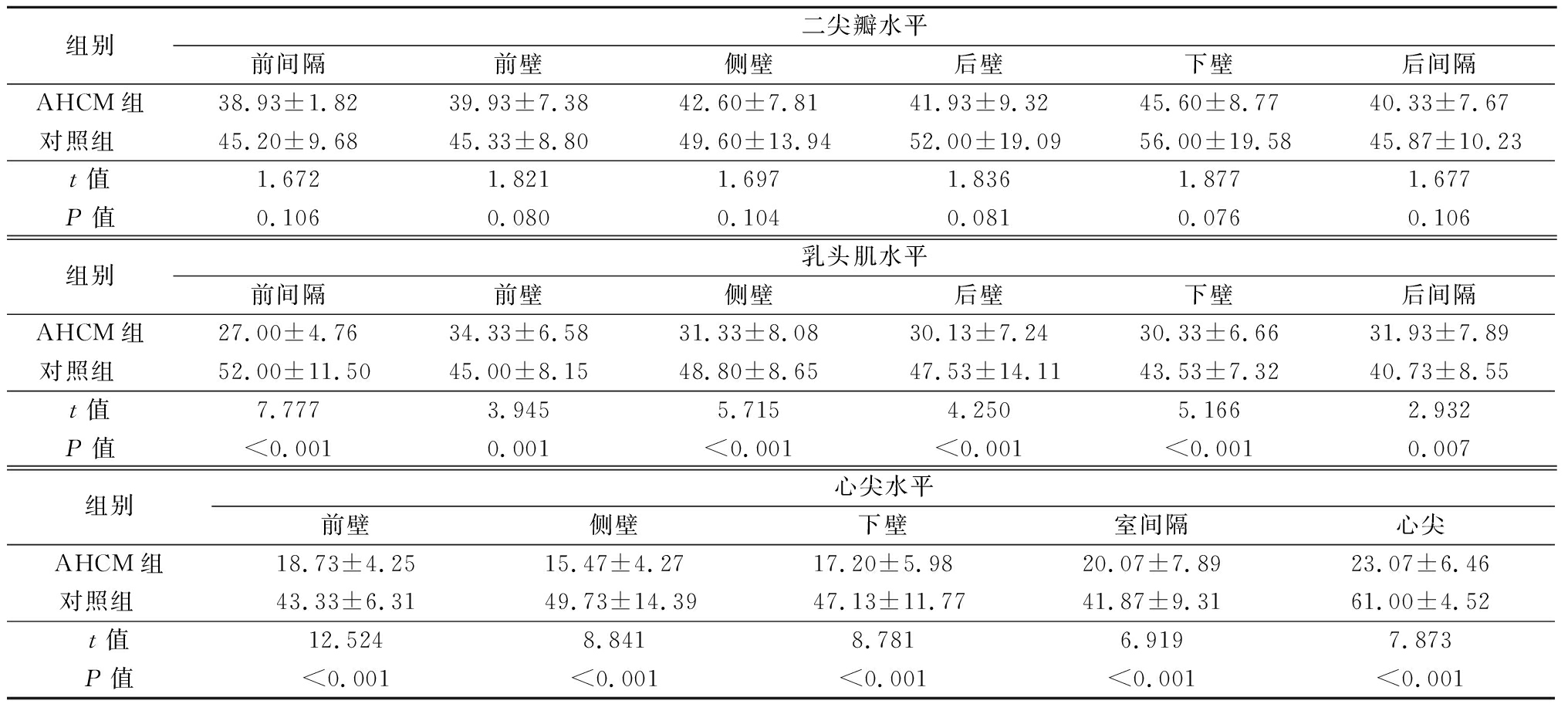
组别二尖瓣水平前间隔前壁侧壁后壁下壁后间隔AHCM组38.93±1.8239.93±7.3842.60±7.8141.93±9.3245.60±8.7740.33±7.67对照组 45.20±9.6845.33±8.8049.60±13.9452.00±19.0956.00±19.5845.87±10.23t值1.6721.8211.6971.8361.8771.677P值0.1060.0800.1040.0810.0760.106组别乳头肌水平前间隔前壁侧壁后壁下壁后间隔AHCM组27.00±4.7634.33±6.5831.33±8.0830.13±7.2430.33±6.6631.93±7.89对照组 52.00±11.5045.00±8.1548.80±8.6547.53±14.1143.53±7.3240.73±8.55t值7.7773.9455.7154.2505.1662.932P值<0.0010.001<0.001<0.001<0.0010.007组别心尖水平前壁侧壁下壁室间隔心尖AHCM组18.73±4.2515.47±4.2717.20±5.9820.07±7.8923.07±6.46对照组 43.33±6.3149.73±14.3947.13±11.7741.87±9.3161.00±4.52t值12.5248.8418.7816.9197.873P值<0.001<0.001<0.001<0.001<0.001
3 讨 论
AHCM多数没有临床症状和体征,在临床诊断中以超声心动图和心电图联合诊断为基础。超声心动图表现以心尖部室壁的不均匀增厚为主,其余室壁无明显增厚;心电图胸前导联,尤其是V3~V5导联呈对称性倒置的巨T波,不能用冠状动脉病变心肌缺血解释,更具有诊断意义。其病理改变以左心室心尖部心肌肥厚为主[2]。一直以来,认为AHCM患者较肥厚型心肌病其他亚型患者预后好。而研究显示,AHCM患者的心血管不良事件发生率为25%~30%[3]。正常人左心室从基底部到心尖部室壁厚度逐渐变薄。AHCM患者的心肌不能满足正常心脏功能的需要,发生代偿性增厚,这种增厚并不是心肌数目的增多,而是心肌细胞的体积增大。由于心肌增厚、心肌纤维排列紊乱、间质纤维化等结构异常,心脏的局部功能已经受损[4]。肥厚的心肌导致心室顺应性降低,通过机体代偿LVEF仍可维持在正常范围[5]。
LVEF反映的是左心室容积的变化,当心肌细胞功能异常不足以导致明显的容积变化时,LVEF就失去了其评估价值。AHCM患者只有到疾病晚期或出现并发症时LVEF才会降低。3D-STI可以反映心肌的形变能力,评估心肌细胞功能受损情况,提供心肌功能障碍的早期信息[6]。
本研究结果显示,AHCM 组整体LS、整体CS、整体AS和整体RS值均低于对照组,LVEF在正常范围。AHCM患者肥厚心肌因冠状动脉微循环障碍,心肌纤维排列紊乱,心肌间质纤维化,导致左心室心肌功能受损,在心肌运动的各个方向上均有体现,这也说明在LVEF明显改变之前,AHCM患者的心肌功能障碍已经出现。左心室壁外层和内层的心肌纤维为不同方向的斜向走行,收缩期的运动形式以纵向运动为主,且心肌纤维对微灌注损伤和间质的纤维化都很敏感,所以在疾病早期,LS即表现为显著减低[7-9]。左心室整体LS和整体CS可以用来预测无冠状动脉狭窄的肥厚型心肌病患者的不良心血管事件,评估预后。AS是3D-STI特有的参数,在左心室收缩时,AS结合了LS和CS的改变,在一定程度上反映了心肌运动,参考意义更大[10-11]。
AHCM组心尖水平各节段的应变值均减低,乳头肌水平及二尖瓣水平则以LS减低最为显著。心肌肥厚部位细胞排列紊乱,使收缩期肌小节缩短率减低,从而使局部应变峰值减低;心肌间质纤维化,心肌细胞重排,心尖水平的LS、CS及AS均明显减低,非肥厚节段局部应变减低与该节段心肌亦存在心肌纤维化有关[12-13]。这也提示心肌功能障碍时,LS改变更加敏感。
彭源等[14]研究显示, HCM组左心室心肌整体LS、整体AS、整体RS均明显低于对照组。本研究结果与之略有差别,考虑可能与所选病例组心肌肥厚节段及肥厚程度不同有关。本研究AHCM组未对心肌肥厚程度进行区分;未考虑β受体阻滞剂等药物对心肌的影响;样本量较小,有待于今后加大样本量进一步研究。
心脏属于三维结构,其运动亦属于三维空间的运动。3D-STI是一项测量心肌运动变形的新技术,应用分析软件自动追踪并对感兴趣区域内心肌变形进行计算,获取运动速度、旋转角度、应变及应变率等多种心肌力学参数,可以定量评价心脏局部及整体功能[15]。3D-STI通过采集心脏三维容积数据,能够全面观察心脏的形态、运动及功能的变化。3D-STI采集图像仅需心尖四腔心一个切面,即可同时提供RS、LS及CS正交应变值,较二维斑点追踪成像的评估更简单,获得的信息更多,更具有全面性,且与磁共振有非常高的相关性[16-17]。3D-STI通过左心室完整、直观的可视化对肥厚心肌进行评估,避免了几何假设所带来的误差,使局灶性肥厚区域的识别更加容易[1]。作为一项新技术,3D-STI亦存在一定的局限性,其影响因素包括图像是否清晰、帧频能否达到标准、心内膜的界限是否清楚、心脏体积过大则图像不完整、心律不齐不能保证图像质量等,这些都需要通过科技的进步而改进。
综上所述,AHCM患者左心室整体收缩功能有不同程度地减低,局部收缩功能的减低以心尖水平最为明显,乳头肌水平亦受累。通过3D-STI对AHCM患者的应变进行测量分析,评价其心肌运动状态,可作为左心室收缩功能评价的一个指标。
[1] Inciardi RM, Galderisi M, Nistri S,et al. Echocardiographic advances in hypertrophic cardiomyopathy:three-dimensional and strain imaging echocardiography[J]. Echocardiogaphy, 2018,35(5):716-726.
[2] 林胜男,阮琴韵.心尖肥厚型心肌病心尖形态学与动力学特征的研究进展[J].中华高血压杂志,2018, 26(4):317-321.
[3] Jan MF,Todaeo MC,Oreto L,et al. Apical hypertrophic cardiomyopathy:present status[J]. Int J Cardiol,2016,222:745-759.
[4] Weidemann F,Störk S,Liu D,et al. Cardiomyopathy of friedreich ataxia[J]. J Neurochem, 2013,126(Suppl 1):88-93.
[5] 江添,刘明辉.实时三平面斑点追踪技术评价肥厚型心肌病左心室整体及节段收缩期纵向应变的研究[J].新医学,2016,47(2):113-116.
[6] Haland TF,Saberniak J,Leren LS,et al. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy[J]. Int J Cardiol,2017,228:900-905.
[7] Smiseth OA,Torp H,Opdahl A,et al. Myocardial strain imaging:how useful is it in clinical decision making?[J]. Eur Heart J,2016,37(15):1196-1207.
[8] Fujimoto K,Inoue K,Saito M,et al. Incremental value of left atrial active function measured by speckle tracking echocardiography in patients with hypertrophic cardiomyopathy[J]. Echocardiography,2018,35(8):1138-1148.
[9] Candan O,Gecmen C,Bayam E,et al. Mechanical dispersion and global longitudinal strain by speckle tracking echocardiography:predictors of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy[J]. Echocardiography, 2017,34(6):835-842.
[10] Ozawa K,Funabash N,Takaoka H,et al. Successful MACE risk stratification in hypertrophic cardiomyopathy patients using different 2D speckle-tracking TTE approaches[J]. Int J Cardiol,2017,228:1015-1021.
[11] Haland TF, Almaas VM,Hasselberg NE,et al. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy[J]. Eur Heart J Cardiovasc Imaging,2016,17(6):613-621.
[12] 刘燕,孙丹丹,杨军.斑点追踪技术评价肥厚型心肌病患者左心室整体及局部收缩功能的临床应用研究进展[J/CD].中华医学超声杂志(电子版),2017,14(11):819-823.
[13] 于欢,姜克新,孙璐,等.三维斑点追踪成像技术评价肥厚型心肌病左心室心肌功能[J].中国医学影像学杂志,2015,23(5):329-333.
[14] 彭源,杨军,孙丹丹,等.三维斑点追踪成像技术评价肥厚型心肌病患者左心室整体和局部收缩功能[J].中国医学影像技术,2014,30(11):1645-1649.
[15] 姚磊,韩若凌.超声斑点追踪成像在心脏疾病中的诊断价值[J].河北医科大学学报,2014,35(2):243-246.
[16] Satriano A, Heydari B, Narous M,et al. Clinical feasibility and validation of 3D principal strain analysis from cine MRI:comparison to 2D strain by MRI and 3D speckle tracking echocardiography[J]. Int J Cardiovasc Imaging,2017,33(12):1979-1992.
[17] Luo HX,Zhou XL,Kou HJ,et al. Improvement of continuous subcutaneous insulin infusion on patients with type 2 diabetes mellitus by 3-dimensional speckle tracking echocardiography[J]. Int J Cardiovasc Imaging,2018,34(3):379-384.