附睾炎是中青年男性最常见的泌尿生殖系统疾病[1]。发病机制是细菌感染从尿道逆行进入男性输精管,进入附睾引发炎症[2-3]。附睾炎不但危害男性生殖健康,而且是男性不孕不育的重要原因之一[4-5]。附睾上皮细胞作为抵御细菌侵入的防护卫士在附睾炎发生发展过程中发挥重要的作用[5],但当机体发生严重感染时,其不受宿主免疫反应调控,造成大量淋巴细胞浸润且产生炎性因子,导致组织损伤[6]。因此,控制附睾上皮细胞的炎性反应是有效治疗附睾炎的方法之一。全反式维甲酸(all-trans-retinoic acid,atRA)是维生素A的中间代谢物, 通过与维甲酸α受体(retinoic acid receptor alpha,RARα)、维甲酸β受体(retinoic acid receptor beta,RARβ)、维甲酸γ受体(retinoic acid receptor gamma,RARγ)结合激活或抑制下游靶基因的转录调控,在动物生长发育过程中发挥重要的作用[7-9]。研究表明,atRA可以通过体液和细胞免疫调节发挥抗炎作用,维持免疫内稳态,调节免疫细胞的分化[10]。同时有证据表明,atRA在多种自身免疫性疾病和反应性疾病中发挥重要作用[11],在生殖系感染时维持上皮细胞功能中扮演重要的角色[12]。atRA与对抗生殖系统病原菌感染密切相关,但是在附睾炎中atRA的作用及抗炎机制尚未明确。本研究以脂多糖(lipopolysaccharide,LPS)诱导的原代小鼠附睾上皮细胞为炎症模型,探讨atRA对附睾上皮细胞中炎性因子的表达调控及分子机制,为治疗附睾炎提供新思路。报告如下。
1 材料与方法
1.1 实验动物与试剂 10~12周雄性C57BL/6J小鼠,体重(25±5) g,购买于河北医科大学实验动物中心。保证12 h光照和12 h黑暗交替规律周期条件,温度22~24 ℃、湿度60%~70%,充足的饲料和水源进行饲养。DMEM培养基(Dulbecco′s modification of Eagle′s medium,DMEM)(Gibico,USA),胎牛血清(fetal bovine serum,FBS)(Gemini,New Zealand),0.25%胰蛋白酶(Gibico,USA),细胞专用磷酸缓冲盐溶液(phosphate buffer saline,PBS)缓冲液(Gibico,USA),Hank′ s 平衡盐溶液(Hank′s balanced salt solution,HBSS)(Gibico,USA), 胶原蛋白酶(Sigma Aldrich, USA),细胞培养用青霉素-链霉素(Beyotime,China),atRA(MCE,USA),LPS(MCE,USA),酶联免疫吸附实验(enzyme-linked immunosorbent assay,ELISA)检测试剂盒(Proteintech,USA),蛋白酶抑制剂(Cell Signaling Technology,USA),兔抗鼠转染转化生长因子β1(transforming growth factor β1,TGF-β1)单克隆抗体(SantaCruz, USA),鼠抗鼠GAPDH单克隆抗体(Cell Signaling Technology,USA),RNA提取试剂TRIZol、RT-PCR试剂盒(Takara,Japan),SYBR快速荧光定量PCR试剂盒(Invitrogen,USA),发光液(ECL Reagent)(BIO-RAD,USA),lipofectamine 2000TM 转染试剂(Invitrogen,USA),Si-TGF-β1干扰RNA(Genma,China),BMS195614(SantaCruz, USA),LE135(SantaCruz, USA)。
1.2 小鼠附睾上皮细胞原代培养 根据Wong[13]和Cuthbert等[14] 的方法培养小鼠睾丸上皮细胞,雄性小鼠处死后,在超净工作台中快速分离小鼠附睾,HBSS冲洗3 次,用手术剪将附睾组织充分剪碎至糜状,加入含有0.25%的胰蛋白酶消化液, 34 ℃消化30 min ;1 000 ×g 离心 5 min,弃去胰蛋白酶后加入含有1 g/L胶原蛋白酶1 mL, 34 ℃消化60 min,800×g离心5 min, 弃去胶原蛋白酶后用DMEM 完全培养基充分洗涤细胞, 弃上清,加入4 mL DMEM 完全培养基, 充分混匀细胞,依次用100和200目不锈钢滤网过滤,将过滤液转移入25 mL 培养瓶中,在34 ℃、5% CO2的培养箱中培养6 h ,待平滑肌细胞等其他非上皮细胞贴壁,上皮细胞尚未贴壁,吸出未贴壁的细胞即为附睾上皮细胞(纯度为90 %以上), 分别种于孔板中,加入DMEM 完全培养基, 在34 ℃、5% CO2 的培养箱中培养, 每3 d换液1次。5 d长成单层细胞后用于后续实验。
1.3 LPS和atAR对附睾上皮细胞的干预实验 将生长状态良好的附睾上皮细胞接种孔板,待细胞贴壁后,更换为含1 %FBS 的DMEM 培养基12 h,分别加入含或不含LPS(10 μg/L)的DMEM 完全培养基和不同浓度的atAR(1,10,100 nmol/L)或相同体积的二甲基亚砜(dimethyl sulfoxide,DMSO),培养24 h,收集用于后续检测,每个样本设置3个重复。
1.4 RAR 拮抗剂BMS195614和RARβ拮抗剂LE135对附睾上皮细胞的干预实验 将生长状态良好的附睾上皮细胞接种孔板,待细胞贴壁后,更换为含1 %FBS 的DMEM 培养基12 h后,分别加入atAR(10 nmol/L)或同体积的DMSO或BMS195614(200 nmol/L) 和RARβ拮抗剂LE135(500 nmol/L),培养24 h,收集用于后续检测,每个样本设置3个重复。
1.5 敲低TGF-β1的附睾上皮细胞的转染实验 附睾上皮细胞生长至密度80%~90%融合后,以0.25%胰蛋白酶溶液消化后弃去,用DMEM培养基(含10%胎牛血清)制备细胞悬液,接种于6孔板中,放入34 ℃、5% CO2的细胞培养箱中培养,待细胞贴壁后,使用Lipofecta mine 2000 对附睾上皮细胞进行Si-TGF-β1干扰RNA的转染,具体步骤参照该 Lipofecta mine 2000 Reagent说明书,转染6 h后进行换液为无血清培养基,根据实验设计分为3组:Control+LPS组(加入10 μg/L LPS)、atRA+Si-Ctl+LPS组(转染Si-Ctl后加入10 μg/L LPS+10 nmol/L atRA)、atRA+TGF-β1+LPS组(转染TGF-β1敲低后加入10 μg/L LPS+10 nmol/L atRA),各组培养24 h后收集细胞用于后续实验。
1.6 荧光定量PCR检测 取收集处理后的附睾上皮细胞,加入1 ml TRIzol试剂及200 μL氯仿提取总RNA,Nanodrop 核酸定量仪检测提取RNA的浓度和纯度。按照Invitrogen荧光定量PCR的M-MLV第一链合成系统说明书进行逆转录制备cDNA。以18S为内参在ABI7500 Fast 实时荧光定量PCR扩增仪中进行荧光定量PCR扩增以检测各组附睾上皮细胞中白细胞介素1β(interleukin-1β,IL-1β)、白细胞介素6(interleukin-6,IL-6)、肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)、转化生长因子β1(transforming growth factor β1,TGF-β1)、RARα、RARβ mRNA的表达水平。扩增引物由上海生工公司合成,引物序列见表1。反应条件:50 ℃预处理2 min,循环1次;95 ℃预变性10 min,循环1次;95 ℃变性15 s,循环40次;60 ℃退火30 s,循环40次;72 ℃延伸30 s,循环40次。各组均设3个复孔。以2-△△Ct法表示各目的基因的相对表达量。
表1 引物序列
Table 1 Primers for real-time PCR
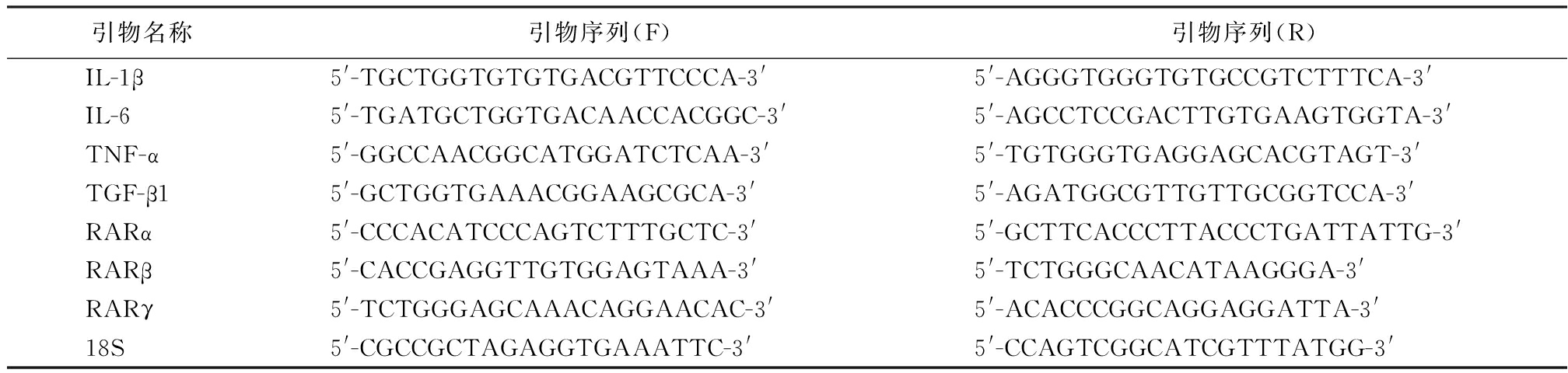
引物名称引物序列(F)引物序列(R)IL-1β5'-TGCTGGTGTGTGACGTTCCCA-3'5'-AGGGTGGGTGTGCCGTCTTTCA-3'IL-65'-TGATGCTGGTGACAACCACGGC-3'5'-AGCCTCCGACTTGTGAAGTGGTA-3'TNF-α5'-GGCCAACGGCATGGATCTCAA-3'5'-TGTGGGTGAGGAGCACGTAGT-3'TGF-β15'-GCTGGTGAAACGGAAGCGCA-3'5'-AGATGGCGTTGTTGCGGTCCA-3'RARα5'-CCCACATCCCAGTCTTTGCTC-3'5'-GCTTCACCCTTACCCTGATTATTG-3'RARβ5'-CACCGAGGTTGTGGAGTAAA-3'5'-TCTGGGCAACATAAGGGA-3'RARγ5'-TCTGGGAGCAAACAGGAACAC-3'5'-ACACCCGGCAGGAGGATTA-3'18S5'-CGCCGCTAGAGGTGAAATTC-3'5'-CCAGTCGGCATCGTTTATGG-3'
1.7 Western Blot检测 附睾上皮细胞处理24 h,离心收集细胞,加入RIPA细胞裂解液,冰上裂解30 min,离心收集上清提取细胞蛋白。BCA法测定细胞总蛋白浓度。各孔取30 μg蛋白上样,于10%聚丙烯酰胺凝胶电泳进行蛋白分离(浓缩胶90 V电压,30 min;分离胶120 V电压,90 min)。将分离后的蛋白电转移(200 mA电流35 min)至PVDF膜。加入5%脱脂奶粉封闭液于摇床上室温封闭2 h。分别加入兔抗鼠TGF-β1和鼠抗鼠GAPDH单克隆抗体(体积稀释比例均为1:1 000)4 ℃反应过夜。次日先以TBST洗膜5次,每次10 min。后加入HRP标记的鼠抗兔或兔抗鼠IgG(体积稀释比例为1:20 000),室温反应1 h。再用TBST洗膜5次,每次10 min。按ECL试剂盒说明进行显影。采用LK5100电化学发光分析系统对蛋白条带灰度值进行分析。
1.8 ELISA检测细胞因子的表达 ①从冰箱中取出细胞因子检测试剂盒恢复至室温;②分别设置标准品孔、空白对照孔和待测样本孔,加入100 μL/孔的相应样品;③ 37 ℃孵育1.5 h;0.01 mol/L PBS洗板3次;④加入生物素标记的抗鼠IL-1β、IL-6、TNF-α抗体工作液100 μL/孔,37 ℃孵育60 min;0.01 mol/L PBS洗板3次;⑤加入ABC工作液100 μL/孔,37 ℃孵育30 min;0.01 mol/L PBS洗板5次;⑥加入90 μL/孔TMB显色剂,37 ℃避光孵育30 min;⑦加入终止液100 μL/孔,终止反应;⑧以空白孔调零,在终止后15 min内,用450 nm波长测量各孔的吸光度(optical density,OD)值;⑨绘制出标准曲线,根据样本的OD值计算出对应的样品浓度。
1.9 统计学方法 采用Graphad 5.0统计软件处理数据。计量资料比较采用t检验、F检验和SNK-q检验。 P<0.05为差异有统计学意义。
2 结 果
2.1 各组小鼠附睾上皮细胞炎性因子mRNA相对表达量比较 LPS组附睾上皮细胞中IL-1β、IL-6和TNF-α mRNA的表达水平明显高于对照组,给予不同浓度的atRA处理后小鼠附睾上皮细胞的炎性因子IL-1β、IL-6和TNF-α mRNA的表达水平明显降低,呈浓度梯度依赖性,差异有统计学意义(P<0.01),见表2。说明LPS可以诱导小鼠附睾上皮细胞炎性因子的表达。
2.2 各组小鼠附睾上皮细胞培养基上清中炎性因子含量比较 LPS组附睾上皮细胞培养基上清中IL-1β、IL-6和TNF-α含量明显高于对照组,给予不同浓度的atRA处理后小鼠附睾上皮细胞培养基上清中IL-1β、IL-6和TNF-α含量明显降低,呈浓度梯度依赖性,差异有统计学意义(P<0.01),见表3。说明atRA可以抑制LPS诱导的小鼠附睾上皮细胞的炎性反应。
表2 各组小鼠附睾上皮细胞炎性因子mRNA相对表达量比较
Table 2 The mRNA relative expression of inflammatory factors in mice![]()
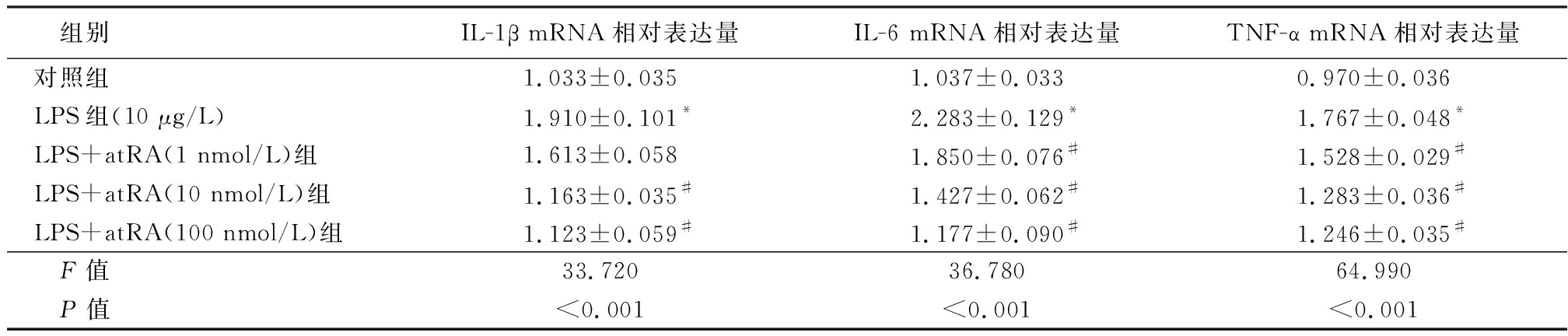
组别IL-1β mRNA相对表达量IL-6 mRNA相对表达量TNF-α mRNA相对表达量对照组1.033±0.0351.037±0.0330.970±0.036LPS组(10 μg/L)1.910±0.101*2.283±0.129*1.767±0.048*LPS+atRA(1 nmol/L)组1.613±0.0581.850±0.076#1.528±0.029#LPS+atRA(10 nmol/L)组1.163±0.035#1.427±0.062#1.283±0.036#LPS+atRA(100 nmol/L)组1.123±0.059#1.177±0.090#1.246±0.035# F值33.72036.78064.990 P值<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与LPS组比较(SNK-q检验)
表3 各组小鼠附睾上皮细胞培养基上清中炎性因子含量比较
Table 3 The content of inflammatory factors in cultured epididymal epithelium![]()
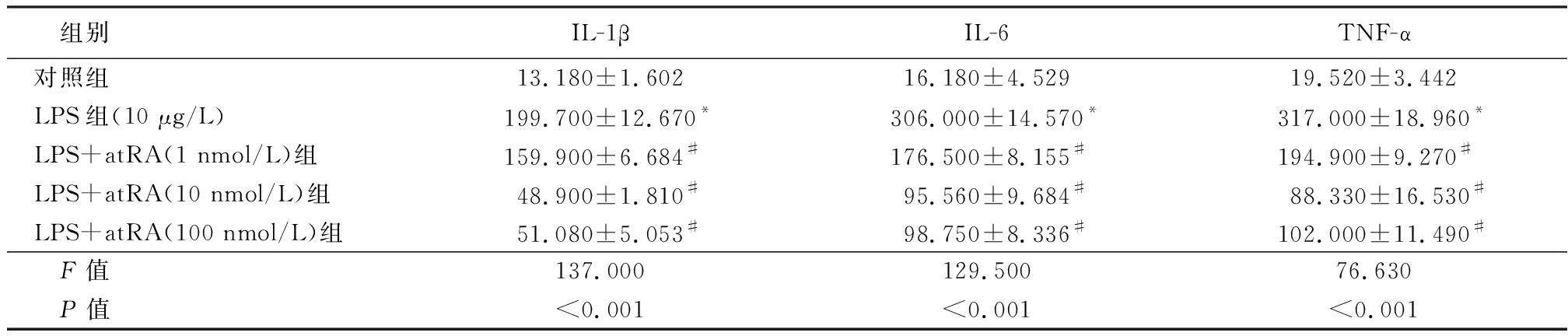
组别IL-1β IL-6 TNF-α对照组13.180±1.60216.180±4.52919.520±3.442LPS组(10 μg/L)199.700±12.670*306.000±14.570*317.000±18.960*LPS+atRA(1 nmol/L)组159.900±6.684#176.500±8.155#194.900±9.270#LPS+atRA(10 nmol/L)组48.900±1.810#95.560±9.684#88.330±16.530#LPS+atRA(100 nmol/L)组51.080±5.053#98.750±8.336#102.000±11.490# F值137.000129.50076.630 P值<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与LPS组比较(SNK-q检验)
2.3 atRA诱导小鼠附睾上皮细胞中TGF-β1的表达 实时定量PCR分析结果显示,LPS组小鼠附睾上皮细胞中TGF-β1 mRNA的表达水平明显低于对照组,给予不同浓度atRA处理后小鼠附睾上皮细胞中TGF-β1 mRNA的表达水平明显升高,呈浓度梯度依赖性,差异有统计学意义(P<0.01),见表4。Western blot分析结果表明,给予10 nmol/L atRA可以显著诱导TGF-β1蛋白水平的表达(图1)。表明atRA可以显著诱导TGF-β1的mRNA和蛋白水平,提示atRA可能通过诱导TGF-β1的表达而发挥抑制LPS诱导的小鼠附睾上皮细胞的炎性反应。
表4 各组小鼠附睾上皮细胞中TGF-β1 mRNA相对表达量比较
Table 4 The mRNA relative expression of TGF-β1 in epididymal epithelium![]()
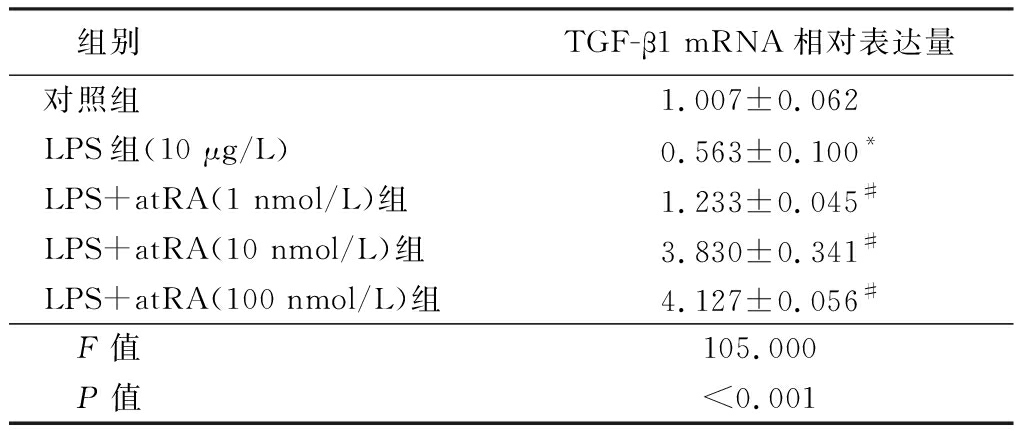
组别TGF-β1 mRNA相对表达量对照组1.007±0.062LPS组(10 μg/L)0.563±0.100*LPS+atRA(1 nmol/L)组1.233±0.045#LPS+atRA(10 nmol/L)组3.830±0.341#LPS+atRA(100 nmol/L)组4.127±0.056# F值105.000 P值<0.001
*P值<0.05与对照组比较 #P值<0.05与LPS组比较(SNK-q检验)
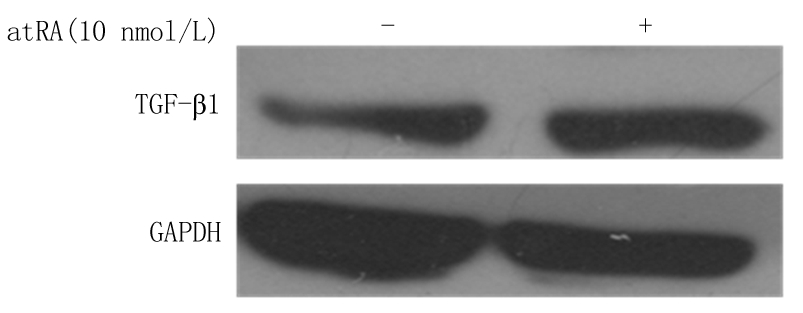
图1 atRA诱导小鼠附睾上皮细胞中TGF-β1 蛋白表达
Figure1 The expression of TGF-βprotein level in mouse epididymal epithelium treated with atRA
2.4 atRA通过上调TGF-β1的表达发挥抑制LPS诱导的小鼠附睾上皮细胞炎性反应 在小鼠附睾上皮细胞中转染TGF-β1的小干扰RNA,敲低细胞中内源性TGF-β1的表达,实时荧光定量PCR结果显示,Si-TGF-β1组TGF-β1 mRNA表达水平明显低于Si-Ctl组,差异有统计学意义(P<0.01)。见表5。Western blot检测结果显示,Si-TGF-β1组TGF-β1蛋白表达水平低于Si-Ctl组(图2),说明转染TGF-β1的小干扰RNA成功降低了细胞内源性TGF-β1的表达。检测炎性因子的表达情况结果显示,atRA+Si-Ctl+LPS组小鼠附睾上皮细胞IL-1β、IL-6和TNF-α mRNA相对表达量低于Control+LPS组,atRA+Si-TGF-β1+LPS组小鼠附睾上皮细胞IL-1β、IL-6和TNF-α mRNA相对表达量明显高于atRA组,差异有统计学意义(P<0.01),见表6。说明敲低TGF-β1可以抵消atRA抑制LPS诱导的附睾上皮细胞的炎性反应。ELISA检测附睾上皮细胞培养液上清中炎性因子的含量,atRA+Si-Ctl+LPS组小鼠附睾上皮细胞培养液上清中IL-1β、IL-6和TNF-α含量低于Control+LPS组,atRA+Si-TGF-β1+LPS组小鼠附睾上皮细胞培养液上清中IL-1β、IL-6和TNF-α含量明显高于atRA组,差异有统计学意义(P<0.01),见表7。证明atRA通过上调TGF-β1的表达发挥抑制LPS诱导的小鼠附睾上皮细胞炎性反应。
表5 转染Si-TGF-β1后小鼠附睾上皮细胞中TGF-β1的表达
Table 5 The expression of TGF-β1 in epididymal epithelium after treating with Si-TGF-β1 ![]()
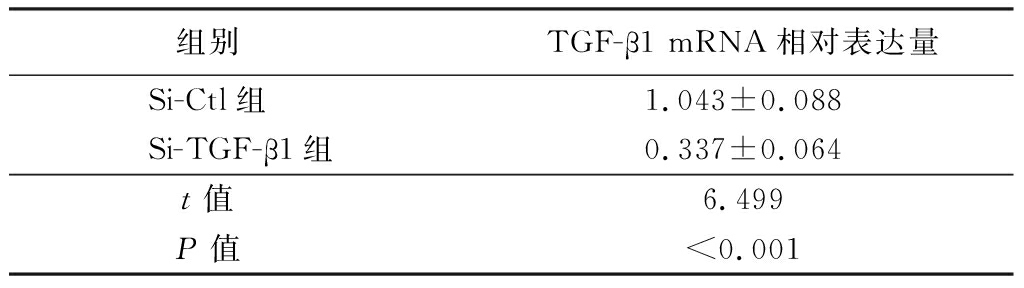
组别 TGF-β1 mRNA相对表达量Si-Ctl组 1.043±0.088Si-TGF-β1组 0.337±0.064 t值 6.499P值 <0.001
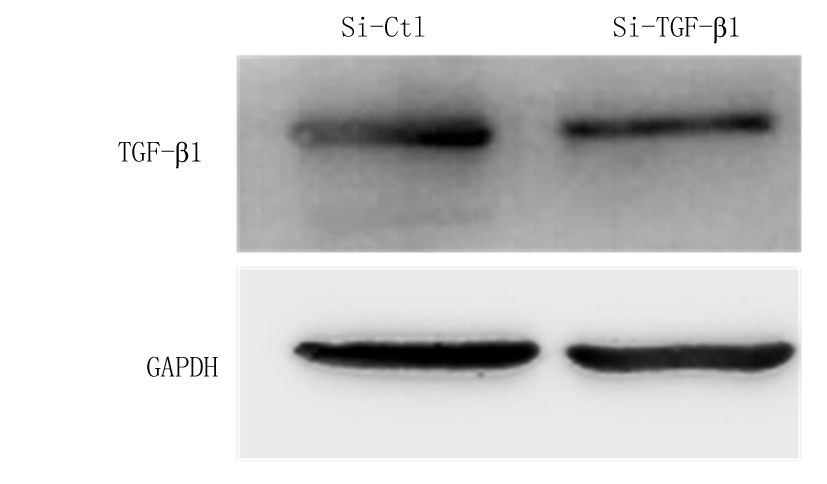
图2 转染Si-TGF-β1后小鼠附睾上皮细胞中TGF-β1的蛋白表达水平
Figure 2 The expression of TGF-βprotein level in mouse epididymal epithelium treated with Si-TGF-β1
表6 敲低TGF-β1给予LPS处理的小鼠附睾上皮细胞的炎性因子的表达变化
Table 6 The expression of inflammatory factors in epididymal epithelium treated with LPS after knocking down TGF-β1 ![]()
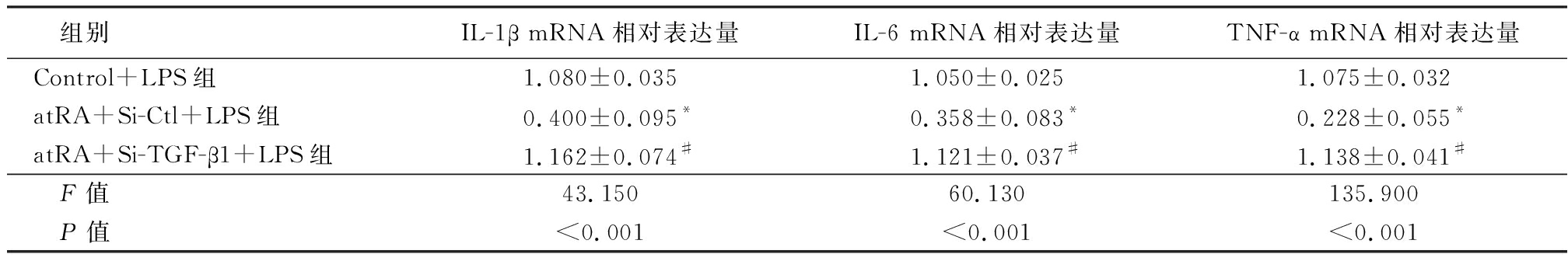
组别IL-1β mRNA相对表达量IL-6 mRNA相对表达量TNF-α mRNA相对表达量Control+LPS组1.080±0.0351.050±0.0251.075±0.032atRA+Si-Ctl+LPS组0.400±0.095*0.358±0.083*0.228±0.055*atRA+Si-TGF-β1+LPS组1.162±0.074#1.121±0.037#1.138±0.041# F值43.15060.130135.900 P值<0.001<0.001<0.001
*P值<0.05与Control+LPS组比较 #P值<0.05与atRA+Si-Ctl+LPS组比较(SNK-q检验)
表7 敲低TGF-β1给予LPS处理的小鼠附睾上皮细胞的炎性因子的含量变化
Table 7 The content of inflammatory factors in epididymal epithelium treated with LPS treated after knocking down TGF-β1 ![]()
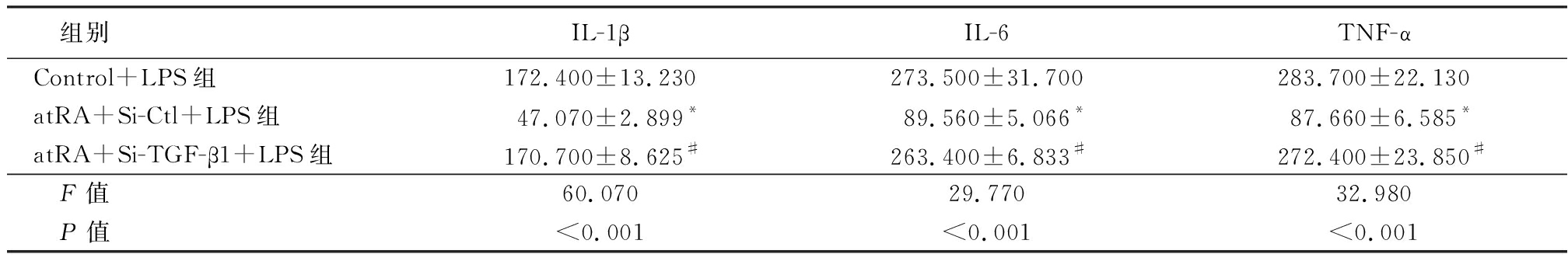
组别IL-1β IL-6 TNF-αControl+LPS组172.400±13.230273.500±31.700283.700±22.130atRA+Si-Ctl+LPS组47.070±2.899*89.560±5.066*87.660±6.585*atRA+Si-TGF-β1+LPS组170.700±8.625#263.400±6.833#272.400±23.850# F值60.07029.77032.980 P值<0.001<0.001<0.001
*P值<0.05与Control+LPS组比较 #P值<0.05与atRA+Si-Ctl+LPS组比较(SNK-q检验)
2.5 atRA可以通过附睾上皮细胞RAR受体诱导TGF-β1的表达 实时荧光定量PCR检测附睾上皮细胞中不同亚型mRNA的表达水平,结果显示,atRA组小鼠附睾上皮细胞中RARα和RARβ mRNA相对表达量明显高于Normal组,差异有统计学意义(P<0.01),2组附睾上皮细胞中RARγ mRNA相对表达量差异无统计学意义(P>0.05),见表8。采用特定的RARα拮抗剂BMS195614和RARβ拮抗剂LE135阻断atRA与RARα和RARβ的结合,进一步观察atRA对TGF-β1的诱导情况,实时荧光定量PCR结果显示,atRA组TGF-β1mRNA相对表达量明显高于Control组,atRA+BMS 195614组TGF-β1mRNA相对表达量明显低于atRA组,差异有统计学意义(P<0.01);atRA+LE135组与atRA组TGF-β1mRNA相对表达量差异无统计学意义(P>0.05),见表9。说明atRA通过结合RARα受体诱导TGF-β1的上调而发挥抑制LPS诱导附睾上皮细胞炎性反应的作用。
表8 atRA处理附睾上皮细胞 后RAR受体的表达
Table 8 RAR receptor expression in epididymal epithelium after atRA treatment![]()
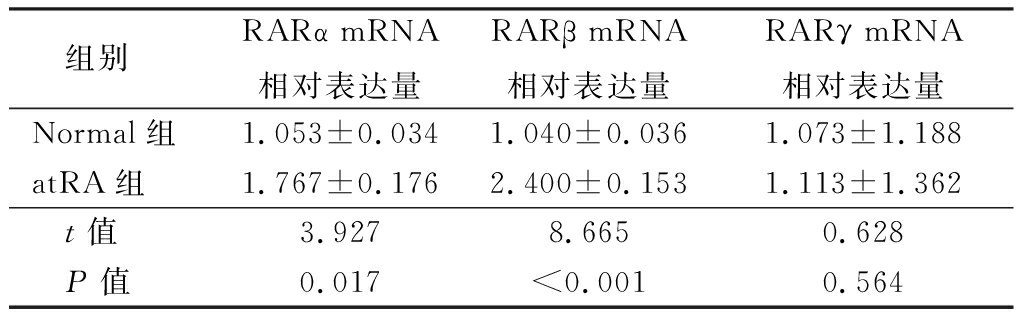
组别RARα mRNA相对表达量RARβ mRNA相对表达量RARγ mRNA相对表达量Normal组1.053±0.0341.040±0.0361.073±1.188atRA组1.767±0.1762.400±0.1531.113±1.362 t值3.9278.6650.628 P值0.017<0.0010.564
表9 给予atRA受体抑制剂后小鼠附睾上皮细胞TGF-β1的表达水平
Table 9 TGF-β1 expression levels after administration of atRA receptor inhibitors in epididymal epithelium![]()
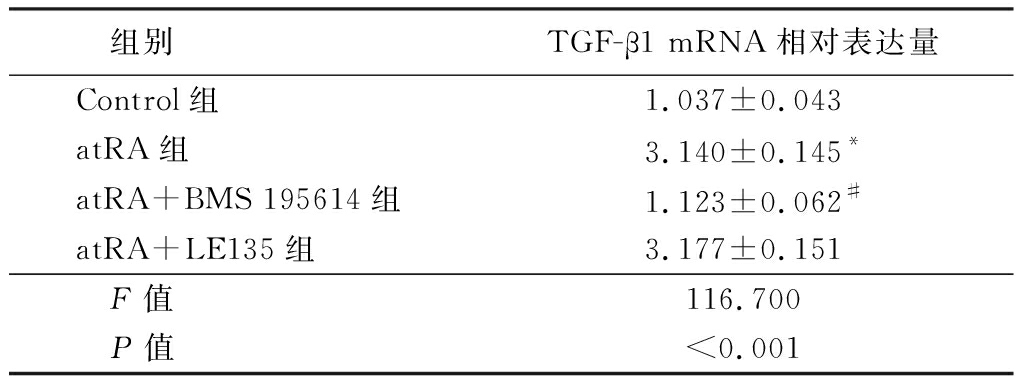
组别TGF-β1 mRNA相对表达量Control组1.037±0.043atRA组3.140±0.145*atRA+BMS 195614组1.123±0.062#atRA+LE135组3.177±0.151 F值116.700 P值<0.001
*P值<0.05与Control组比较 #P值<0.05与atRA组比较(SNK-q检验)
3 讨 论
附睾上皮细胞是附睾抵御病原微生物侵入的重要防线。然而,当机体发生严重细菌感染时,诱导机体产生大量细胞因子,破坏附睾免疫微环境,引起附睾功能损伤,导致男性不孕不育[3]。 但是在附睾炎发生过程中,干扰附睾功能的细胞因子及附睾上皮细胞在炎性反应中的机制尚不明确。因此,为明确附睾上皮细胞在炎性反应中细胞因子的表达变化,本研究以LPS处理原代培养小鼠附睾上皮细胞模拟体外感染模型,发现附睾上皮细胞在LPS处理后能产生大量炎症因子IL-1β、IL-6和TNF-α,说明LPS诱导的附睾上皮细胞产生炎性反应。
维生素A及其中间代谢物通过调节机体免疫反应对多种疾病的发生发展具有良好的控制作用,如类风湿关节炎、1型糖尿病、溃疡性结肠炎等[15-17]。atRA可以通过体液和细胞免疫调节维持免疫内稳态和调节免疫细胞的分化,发挥重要的抗炎、抗感染的作用。但其在附睾炎中发挥抗炎作用的具体调控分子机制并不明确。本研究结果显示,给予LPS处理的附睾上皮细胞不同浓度atRA处理后,atRA可以显著抑制有LPS诱导的附睾上皮细胞中IL-1β、IL-6、TNF-α的表达,促进具有抗炎作用的TGF-β1的表达,说明atRA在附睾上皮细胞中具有显著的抗炎作用。
atRA主要通过RARs(包括RARα、RARβ、RARγ)介导发挥重要的生物学功能。研究报道,在附睾和附睾上皮细胞中RARα和RARβ受体起主要作用[18]。本研究结果表明,给予 atRA刺激的附睾上皮细胞显著增加了RARα和RARβ mRNA的表达。为了明确atRA是通过RARs受体介导而诱导TGF-β1的表达,进一步给予RARα受体抑制剂BMS195641,结果表明后者可显著逆转atRA的作用,而给予RARβ抑制剂LE315并不影响atRA对TGF-β1的诱导作用,说明atRA对于TGF-β1的诱导作用主要是通过RARα受体介导的。atRA在附睾上皮细胞可以显著增加RARα和RARβ mRNA的表达,但只有RARα介导atRA对TGF-β1具有诱导作用,RARβ可能不介导TGF-β1的表达,而介导其它信号通路的活化或分子的调控,具体的下游活化分子是下一步研究的重点,说明atRA可能通过与不同的RARs受体结合诱导不同的下游分子信号活化,发挥不同的生物学作用。提示,深入研究atRA在附睾上皮细胞炎性反应中的重要作用能为治疗附睾炎提供新的思路。
[1] McConaghy JR,Panchal B. Epididymitis:an overview[J]. Am Fam Physician,2016,94(9):723-726.
[2] Hongo H,Kikuchi E,Matsumoto K,et al. Novel algorithm for management of acute epididymitis[J]. Int J Urol,2017,24(1):82-87.
[3] Lynch S. Acute epididymitis[J]. JAAPA,2018,31(3):50-51.
[4] Ge WB,Xiao LF,Duan HW,et al. Melatonin protects against lipopolysaccharide-induced epididymitis in sheep epididymal epithelial cells in vitro[J]. Immunol Lett,2019,214:45-51.
[5] Silva EJR,Ribeiro CM,Mirim AFM,et al. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis[J]. Sci Rep,2018,8(1):103.
[6] Song X,Lin NH,Wang YL,et al. Comprehensive transcriptome analysis based on RNA sequencing identifies critical genes for lipopolysaccharide-induced epididymitis in a rat model[J]. Asian J Androl,2019,21(6):605-611.
[7] Orfali N,Shan-Krauer D,O′Donovan TR,et al. Inhibition of UBE2L6 attenuates ISGylation and impedes ATRA-induced differentiation of leukemic cells[J].Mol Oncol,2019[Epub ahead of print].
[8] Ghyselinck NB,Duester G. Retinoic acid signaling pathways[J]. Development,2019,146(13).pii:dev167502.
[9] Fischer S. Pattern recognition receptors and control of innate immunity:role of nucleic acids[J]. Curr Pharm Biotechnol,2018,19(15):1203-1209.
[10] Oliveira LM,Teixeira FME,Sato MN. Impact of retinoic acid on immune cells and inflammatory diseases[J]. Mediators Inflamm,2018,2018:3067126.
[11] Abdelhamid L,Luo XM. Retinoic acid,leaky gut,and autoimmune diseases[J]. Nutrients,2018,10(8).pii:E1016.
[12] Jauregui EJ,Mitchell D,Topping T,et al. Retinoic acid receptor signaling is necessary in steroidogenic cells for normal spermatogenesis and epididymal function[J]. Development,2018,145(13).pii:dev160465.
[13] Wong PY. Mechanism of adrenergic stimulation of anion secretion in cultured rat epididymal epithelium[J]. Am J Physiol,1988,254(1 Pt 2):F121-F133.
[14] Cuthbert AW,Wong PY. Electrogenic anion secretion in cultured rat epididymal epithelium[J]. J Physiol,1986,378:335-345.
[15] Parastouei K,Mirshafiey A,Eshraghian MR,et al. The effect of 1,25(OH)2 D3(calcitriol) alone and in combination with all-trans retinoic acid on ROR-γt,IL-17,TGF-β,and FOXP3 gene expression in experimental autoimmune encephalomyelitis[J]. Nutr Neurosci,2018,21(3):210-218.
[16] Tamaki M,Tominaga T,Fujita Y,et al. All-trans retinoic acid suppresses bone morphogenetic protein 4 in mouse diabetic nephropathy through a unique retinoic acid response element[J]. Am J Physiol Endocrinol Metab,2019,316(3):E418-E431.
[17] Ye X,Wu H,Sheng L,et al. Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling[J]. Nat Commun,2019,10(1):1463.
[18] Peer NR,Law SM,Murdoch B,et al. Germ cell-specific retinoic acid receptor α functions in germ cell organization,meiotic integrity,and spermatogonia[J]. Endocrinology,2018,159(9):3403-3420.