青春期是个体从儿童逐渐向成人转变的重要过渡时期[1],乳房发育是正常女童青春期开始的标志[2],由于乳房Tanner分期简便、直观,所以被临床医师广泛应用于青春期各阶段的评估[3-4]。但通过对乳房的观察及触诊得出的评估主观性强,应用过程中存在一定的限制。特别是现阶段,肥胖儿童发病率逐渐增高[5-6],肥胖女孩由于脂肪组织堆积,导致触诊时乳房腺体组织及乳房轮廓模糊不清,无法实现准确分期。乳腺超声能更客观地对乳腺组织的形态结构和体积进行评估,目前已被广泛应用于乳腺疾病的诊疗过程[7-8],但却很少用于青春期发育的评估。有学者提出基于乳腺超声的5个乳房发育阶段分级,可以明确乳腺组织学变化,对青春期发育的评估更具客观性[9],但由于临床Tanner分期更实用、便捷,目前乳腺超声还没有被常规应用于青春期乳房发育的评估。笔者通过探讨乳腺超声分级与生长发育有关参数的相关性、分级间的差异性、Tanner分期与乳腺超声分级的一致性,旨在明确青春期发育女童乳腺、卵巢、子宫超声及骨龄和性发育状况的关系及乳腺超声对女童青春期发育评估的意义。
1 资料与方法
1.1 一般资料 选取2016年1月—2018年5月在河北医科大学第二医院门诊接受盆腔及乳腺超声检查以评价生长发育的女童128例,年龄≥6岁,均除外影响下丘脑-垂体-性腺轴的器质性疾病。其中因盆腔超声卵巢或子宫显示不清、无骨龄或促黄体生成素(luteinizing hormone,LH)、卵泡刺激素(follicle stimulating hormone,FSH)、雌二醇(estradiol,E2) 等实验室数据记录不全者18例被排除。所有受试者均按照临床Tanner分期评估乳房发育情况,测量身高、体重,计算体重指数(body mass index,BMI),做乳腺及盆腔超声检查,根据乳腺超声结果进行乳腺超声分级,拍摄左手正位片采用G-P图谱法判读骨龄,采静脉血测LH、FSH及E2。
本研究经医院伦理委员会批准,所有患儿家属同意并签署知情同意书。
1.2 方法
1.2.1 Tanner分期 Tanner分期将乳房发育分为5期[3]:Ⅰ期为青春前乳房,仅见乳头稍突出;Ⅱ期为乳芽期,乳晕增大着色,乳晕和乳头微隆起,乳核直径不超过乳晕;Ⅲ期为乳房和乳晕进一步增大,乳房大小超过乳晕,两者融合突起;Ⅳ期为乳晕和乳头突出于乳房之上,形成第二个隆起;Ⅴ期为成熟期,乳头突起,乳晕回缩,乳晕和乳房又连续成一个半球形的大隆起(存在乳房不对称时,以发育更明显的一侧进行评估)。
1.2.2 临床、骨龄和性激素资料 由儿科内分泌医师测量,身高测量脱鞋取直立平视位,体重测量时受试者脱鞋,穿轻便衣服。所有受试者身高(精确到0.1 cm)、体重(精确到0.1 kg),计算BMI,骨龄(精确到0.1岁),采集所有受试者清晨空腹静脉血4 mL,采用免疫化学发光法测定血清LH、FSH和E2水平。
1.2.3 乳腺及盆腔超声 所有受试者检查前需饮水,使膀胱充分充盈,检查时取仰卧位,由同一名有经验的超声科医师操作Philips iu22型超声诊断仪,应用频率为10 MHz的探头,以乳头为中心,从顺/逆时针方向连续转动检查乳房;更换频率约7.5 MHZ的探头,于下腹正中耻骨联合上方对受试者内生殖器进行测量与观察。
1.2.4 乳腺超声分级 超声将乳房发育分5级[9]:A级为处于青春期前,乳晕后可见轻度不均匀组织,未见明显低回声结节;B级为乳晕后可见直径<1 cm圆形,边缘呈线性的低回声结节(乳芽);C级为乳晕后低回声结节(乳芽)增大,直径≥1 cm,伴或不伴有高回声腺体组织;D级为乳晕周围高回声腺体组织及中央低回声结节进一步增大,且呈分枝状,伴皮下脂肪组织堆积;E级为乳晕周围高回声腺体组织呈三角形伴皮下脂肪组织,无低回声中心结节(存在乳房不对称时,以发育更明显的一侧进行评估)。
1.3 统计学方法 应用SPSS 23.0统计软件分析数据。正态分布的计量资料比较采用单因素方差分析和SNK-q检验,非正态分布的计量资料比较采用秩和检验,相关性采用Pearson相关分析和Spearman相关性分析。P<0.05为差异有统计学意义。
2 结 果
2.1 乳腺超声分级与临床资料及辅助检查等参数相关性分析 相关性分析显示乳腺超声分级与年龄(r=0.621,P<0.001)、骨龄(r=0.707,P<0.001)、BMI(r=0.319,P=0.001)、E2(r=0.297,P=0.002) 、LH(r=0.317,P=0.001)、子宫体积(r=0.744,P<0.001)、子宫长径(r=0.709,P<0.001)、卵巢体积(左右卵巢的平均体积)(r=0.639,P<0.001)呈正相关,与FSH无明显相关性(r=0.169,P=0.078)。
2.2 乳腺超声分级组间临床资料及辅助检查等参数比较 受试女童110例按乳腺超声分为A级23例(20.9%)、B级46例(41.8%)、C级18例(16.4%)、D级5例(4.5%)、E级18例(16.4%)。C组、D组、E组年龄、骨龄、BMI、子宫体积、子宫长径和卵巢体积高于或大于A组和B组,E组E2水平高于A组和B组,D组、E组LH水平高于A组和B组,差异有统计学意义(P<0.05)。见表1。
表1 乳腺超声分级间参数的比较
Table 1 Comparison of parameters in different ultrasound grade of breast [M(QR)]
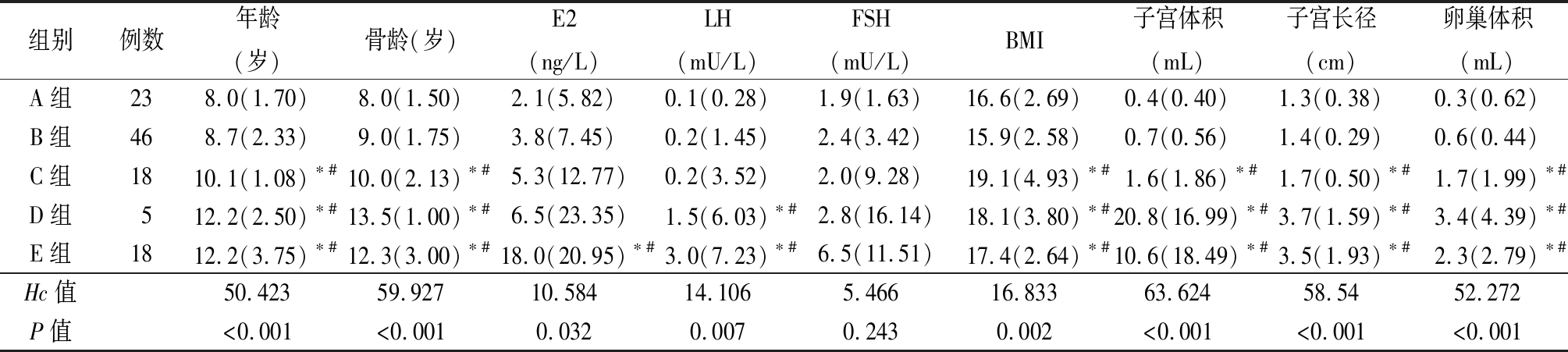
组别例数年龄(岁)骨龄(岁)E2(ng/L)LH(mU/L)FSH(mU/L)BMI子宫体积(mL)子宫长径(cm)卵巢体积(mL)A组238.0(1.70)8.0(1.50)2.1(5.82)0.1(0.28)1.9(1.63)16.6(2.69)0.4(0.40)1.3(0.38)0.3(0.62)B组468.7(2.33)9.0(1.75)3.8(7.45)0.2(1.45)2.4(3.42)15.9(2.58)0.7(0.56)1.4(0.29)0.6(0.44)C组1810.1(1.08)∗#10.0(2.13)∗#5.3(12.77)0.2(3.52)2.0(9.28)19.1(4.93)∗#1.6(1.86)∗#1.7(0.50)∗#1.7(1.99)∗#D组512.2(2.50)∗#13.5(1.00)∗#6.5(23.35)1.5(6.03)∗#2.8(16.14)18.1(3.80)∗#20.8(16.99)∗#3.7(1.59)∗#3.4(4.39)∗#E组1812.2(3.75)∗#12.3(3.00)∗#18.0(20.95)∗#3.0(7.23)∗#6.5(11.51)17.4(2.64)∗#10.6(18.49)∗#3.5(1.93)∗#2.3(2.79)∗#Hc值50.42359.92710.58414.1065.46616.83363.62458.5452.272P值<0.001<0.0010.0320.0070.2430.002<0.001<0.001<0.001
*P值<0.05与A组比较 #P值<0.05与B组比较(秩和检验)
2.3 根据乳腺超声分级比较TannerⅡ期临床资料及辅助检查参数 110例受试者中临床TannerⅡ期女童60例(54.5%),按乳腺超声分级,A级15例(25.0%)、B级38例(63.3%)、C级7例(11.7%)。3组间E2、子宫体积、子宫长径及卵巢体积差异均有统计学意义(P<0.05),且随着超声分级的增加上述参数水平逐渐增加。而年龄、骨龄、LH、FSH、BMI差异无统计学意义(P>0.05)。见表2。
表2 TannerⅡ期女童乳腺超声分级间参数比较
Table 2 Comparison of parameters of girls with breast development stage in Tanner Ⅱ in different breast ultrasound grades [M(QR)]
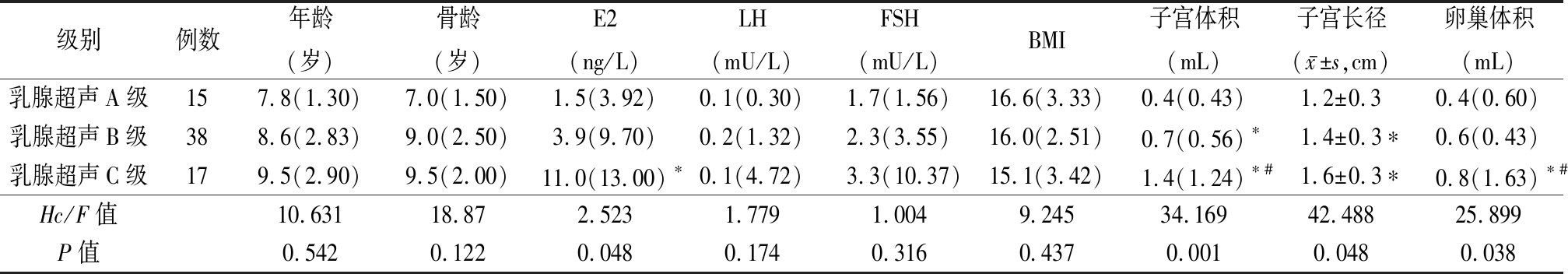
级别例数年龄(岁)骨龄(岁)E2(ng/L)LH(mU/L)FSH(mU/L)BMI子宫体积(mL)子宫长径(x-±s,cm)卵巢体积(mL)乳腺超声A级157.8(1.30)7.0(1.50)1.5(3.92)0.1(0.30)1.7(1.56)16.6(3.33)0.4(0.43)1.2±0.30.4(0.60)乳腺超声B级388.6(2.83)9.0(2.50)3.9(9.70)0.2(1.32)2.3(3.55)16.0(2.51)0.7(0.56)∗1.4±0.3∗0.6(0.43)乳腺超声C级179.5(2.90)9.5(2.00)11.0(13.00)∗0.1(4.72)3.3(10.37)15.1(3.42)1.4(1.24)∗#1.6±0.3∗0.8(1.63)∗#Hc/F值10.63118.872.5231.7791.0049.24534.16942.48825.899P值0.5420.1220.0480.1740.3160.4370.0010.0480.038
*P值<0.05与乳腺超声A级比较 #P值<0.05与乳腺超声B级比较(秩和检验或SNK-q检验)
2.4 根据Tanner分期比较乳腺超声分级临床资料及辅助检查参数 所有受试者中乳腺超声B级女童46例(41.8%),将B级女童按Tanner分期,Ⅰ期5例(10.9%)、Ⅱ期36例(78.2%)、Ⅲ期 5例(10.9%)。3组间年龄、E2、LH、FSH、BMI、子宫体积、子宫长径及卵巢体积差异均无统计学意义(P>0.05),TannerⅢ期组骨龄大于TannerⅠ期组骨龄,差异有统计学意义(P<0.05)。见表3。
表3 乳腺超声B级女童Tanner分期间参数比较
Table 3 Comparison of parameters of girls with breast ultrasound grade B in different Tanner Stages [M(QR)]
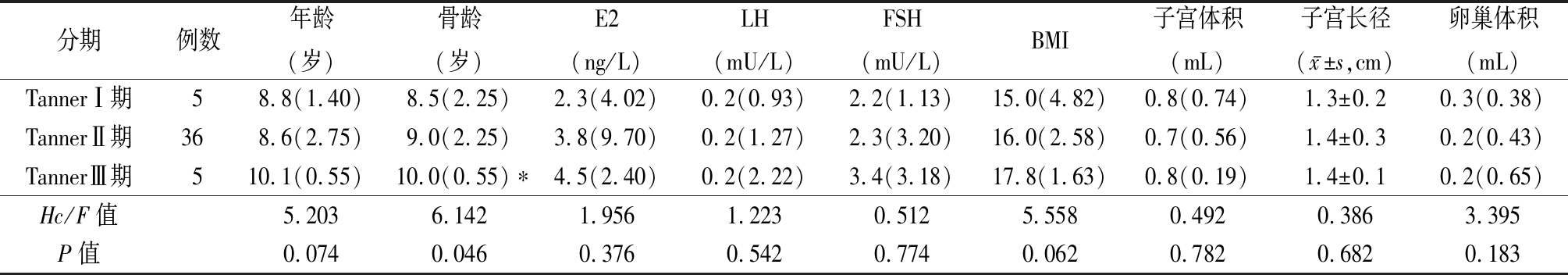
分期例数年龄(岁)骨龄(岁)E2(ng/L)LH(mU/L)FSH(mU/L)BMI子宫体积(mL)子宫长径(x-±s,cm)卵巢体积(mL)TannerⅠ期58.8(1.40)8.5(2.25)2.3(4.02)0.2(0.93)2.2(1.13)15.0(4.82)0.8(0.74)1.3±0.20.3(0.38)TannerⅡ期368.6(2.75)9.0(2.25)3.8(9.70)0.2(1.27)2.3(3.20)16.0(2.58)0.7(0.56)1.4±0.30.2(0.43)TannerⅢ期510.1(0.55)10.0(0.55)∗4.5(2.40)0.2(2.22)3.4(3.18)17.8(1.63)0.8(0.19)1.4±0.10.2(0.65)Hc/F值5.2036.1421.9561.2230.5125.5580.4920.3863.395P值0.0740.0460.3760.5420.7740.0620.7820.6820.183
*P值<0.05与TannerⅡ期比较(秩和检验或SNK-q检验)
3 讨 论
随着社会经济、环境和观念的改变,越来越多的家长开始重视儿童的生长发育[10-11],而乳房发育又是女孩迈入青春期的第一步,直观的Tanner分期有时不能完全确定乳房发育情况,甚至可能导致临床医师对儿童发育状况的错误判断,以往的研究也尝试应用影像学检查来判断乳房发育情况[12-13],其中乳腺超声具有对乳房发育及疾病诊断敏感度高[14-16],检查费用低,没有辐射风险等优点。有学者提出依据超声将乳房从发育到成熟分为从A到E的5个阶段[9,17]。
围青春期下丘脑对促性腺激素释放激素(gonadotropin-releasing hormone,GnRH)的负反馈敏感性降低,下丘脑被激发不断释放GnRH,导致垂体LH和FSH分泌增加,促进性腺合成和分泌性激素,最终诱发乳芽增大,乳房腺体形成,子宫及卵巢增大,阴毛、腋毛出现,阴道出血,性发育逐渐成熟[18]。骨龄的增长也与雌激素作用于生长板有关[19]。本研究中乳腺超声分级与受试者子宫体积、子宫长径及卵巢体积、E2、LH、骨龄均存在正相关性。这与之前性激素水平升高与第二性征发育及骨龄的增长存在正相关的研究观点一致[20-21]。
乳腺超声从A到E的5个等级是乳房从青春期之前到性发育成熟的一个连续性的渐变过程[22],本次研究中超声的5个等级间骨龄、子宫体积、子宫长径、卵巢体积、E2和LH等与青春期发育有关的参数差异均存在统计意义(P<0.05),且参数水平随分级的增高而逐渐上升,这也进一步证明了乳腺超声的等级可以对乳房从发育到成熟的变化过程进行评估。
Tanner分期被广泛应用于青春期女孩乳房发育的评估。然而,这种分期方法仅基于乳房的外观及触诊,没有考虑到发育的个体差异及脂肪堆积区域可能被误认为是真正的乳房萌芽等其他因素。而且以现代儿童饮食变化的趋势,肥胖在儿童中的发病率逐渐增长[23],仅通过物理的手段很难辨别是否存在真正的乳房发育。因此,Tanner分期的准确性在某些情况下值得怀疑。研究表明乳房的临床Tanner分期在超声上表现出不同的成熟程度,以处于青春期初期成熟程度的TannerⅡ期女童为例,B级以上的超声分级可能同雌激素暴露程度高。同样,也可能观察到乳腺成熟度较低的超声分期,特别是将肥胖或超重女童的脂肪堆积错误判断为乳房的早期发育。而对于那些已经出现乳房发育的女童,发育早期由于腺体及其周围组织不易被区分,乳腺不易被触及,反而不能在早期被发现。在乳房发育过程中,部分女童也可能由于体重增长快,局部脂肪堆积导致脂肪组织下的腺体轮廓触诊不清,造成乳房发育分期高于实际乳腺发育水平,而被误认为青春期发育快速进展。
本研究中临床评估为TannerⅡ期的女童乳腺超声分级与Tanner分期不完全一致,其中A级15例(25.0%)、B级38例(63.3%)、C级7例(11.7%),且3个亚组的反应青春期发育的参数(E2、子宫体积、子宫长径及卵巢体积)差异均有统计学意义(P<0.05),且E2、子宫体积、子宫长径及卵巢体积均随乳腺超声分级的增加而显著增加。所有受试者中乳腺超声B级的女童46例(41.8%),Tanner分期也不完全一致,其中TannerⅠ期5例(10.9%)、Ⅱ期36例(78.2%)、Ⅲ期5例(10.9%),3组年龄、E2、LH、FSH、BMI、子宫体积、子宫长径及卵巢体积差异均无统计学意义(P>0.05)。进一步表明乳腺超声分级具有客观性和准确的优势,同Calcaterra等[24]认为超声检查对乳房发育分级比Tanner分期更能反映乳房成熟程度的研究相一致。
然而,本研究也存在局限性,受试女孩相对偏瘦,110例女童中仅有10例(11%)为肥胖儿,其中Tanner分期和乳腺超声评级一致6例,乳腺超声分级低于Tanner分期2例,超声分级高于Tanner分期2例。因此无法评估在较高的BMI分类中Tanner分期错误的风险是否增加。本研究对象的数量不足以评价超声分级评估在女孩乳房发育过程中是必要的常规检查还是选择性检查。
总之,乳腺超声检查是一种简单、可重复、定量测定乳房形态结构的方法。与临床实践中传统使用的Tanner分期相比,可视化乳房的青春期发育阶段,并确保更准确的信息。乳腺超声分级与骨龄、子宫体积、子宫长径、卵巢体积存在明显正相关,预期乳腺超声检查可以减少临床诊断的困境,作为一种高度有效和准确的替代方法,尤其适用于肥胖者和乳腺分期可疑者,在特定受试者人群中的常规使用应得到更多研究支持。
[1] Wood CL,Lane LC,Cheetham T. Puberty:Normal physiology(brief overview)[J]. Best Pract Res Clin Endocrinol Metab,2019,33(3):101265.
[2] Kaplowitz P,Bloch C. Evaluation and referral of children with signs of early puberty[J]. Pediatrics,2016,137(1):10.
[3] Marshall WA,Tanner JM. Variations in pattern of pubertal changes in girls[J]. Arch Dis Child,1969,44(235):291-303.
[4] Klein DA,Emerick JE,Sylvester JE,et al. Disorders of puberty:an approach to diagnosis and management[J]. Am Fam Physician,2017,96(9):590-599.
[5] Schell LM,Gallo MV,Pfeiffer S,et al. Trends in height,weight,BMI,skinfolds,and measures of overweight and obesity from 1979 through 1999 among American Indian Youth:The Akwesasne Mohawk[J]. Int J Obes(Lond),2020,44(3):656-663.
[6] Sun G,Jia G,Peng H,et al. Trends of childhood obesity in China and associated factors[J]. Clin Nurs Res,2015,24(2):156-171.
[7] Chung EM,Cube R,Hall GJ,et al. From the archives of the AFIP:breast masses in children and adolescents:radiologic-pathologic correlation[J]. Radiographics,2009,29(3):907-931.
[8] Lilge L,Terry MB,Walter J,et al. Non-invasive optical spectroscopic monitoring of breast development during puberty[J]. Breast Cancer Res,2017,19(1):12.
[9] Yüce Ö,Sevinç D. Ultrasonographic assessment of pubertal breast development in obese children:compliance with the clinic[J].J Pediatr Endocrinol Metab,2018,31(2):137-141.
[10] Farello G,Altieri C,Cutini M,et al. Review of the literature on current changes in the timing of pubertal development and the incomplete forms of early puberty[J]. Front Pediatr,2019,7(5):147.
[11] Ashley F. Watchful waiting doesn't mean no puberty blockers,and moving beyond watchful waiting[J]. Am J Bioeth,2019,19(6):W3-W4.
[12] Fugl L,Hagen CP,Mieritz MG,et al. Glandular breast tissue volume by magnetic resonance imaging in 100 healthy peripubertal girls:evaluation of clinical tanner staging[J]. Pediatr Res,2016,80(4):526-530.
[13] Fortes CM,Goldberg TB,Kurokawa CS,et al. Relationship between chronological and bone ages and pubertal stage of breasts with bone biomarkers and bone mineral density in adolescents[J].J Pediatr(Rio J),2014,90(6):624-631.
[14] Gao Y,Saksena MA,Brachtel EF,et al. How to approach breast lesions in children and adolescents[J]. Eur J Radiol,2015,84(7):1350-1364.
[15] Lee EJ,Chang YW,Oh JH,et al. Breast lesions in children and adolescents:diagnosis and management[J]. Korean J Radiol,2018,19(5):978-991.
[16] 李媛媛.超声应变率成像技术对急性期川崎病患儿左心室局部收缩功能的评价[J].河北医科大学学报,2015,36(4):480-482.
[17] García CJ,Espinoza A,Dinamarca V,et al. Breast US in children and adolescents[J]. Radiographics,2000,20(6):1605-1612.
[18] Dagklis T,Ravanos K,Makedou K,et al. Common features and differences of the hypothalamic-pituitary-gonadal axis in male and female[J]. Gynecol Endocrinol,2015,31(1):14-17.
[19] Crocker MK,Gourgari E,Lodish M,et al. Use of aromatase inhibitors in large cell calcifying sertoli cell tumors:effects on gynecomastia,growth velocity,and bone age[J]. J Clin Endocrinol Metab,2014,99(12):E2673-2680.
[20] Kelsey TW,Ginbey E,Chowdhury MM,et al. A Validated normative model for human uterine volume from birth to age 40 years[J]. PLoS One,2016,11(6):e0157375.
[21] Xu YQ,Li GM,Li Y. Advanced bone age as an indicator facilitates the diagnosis of precocious puberty[J]. J Pediatr(Rio J),2018,94(1):69-75.
[22] Binay C,Simsek E,Bal C. The correlation between GnRH stimulation testing and obstetric ultrasonographic parameters in precocious puberty[J]. J Pediatr Endocrinol Metab,2014,27(11/12):1193-1199.
[23] Chen C,Zhang Y,Sun W,et al. Investigating the relationship between precocious puberty and obesity:a cross-sectional study in Shanghai,China[J]. BMJ Open,2017,7(4):e014004.
[24] Calcaterra V,Sampaolo P,Klersy C,et al. Utility of breast ultrasonography in the diagnostic work-up of precocious puberty and proposal of a prognostic index for identifying girls with rapidly progressive central precocious puberty[J]. Ultrasound Obstet Gynecol,2009,33(1):85-91.