化脓性脑膜炎(purulent meningitis,PM)是一种严重的颅内感染性疾病。尽管目前在PM的诊断和治疗方面已取得较大的进展,但仍有较高的病死率及致残率。因此,早期诊断、早期准确评估病情轻重对预后显得尤为重要。目前临床对PM患儿病情严重程度的判断主要依据临床表现、影像学检查和格拉斯哥昏迷评分等,尚缺乏客观可量化的评价指标。尤其对于低龄患儿,其症状不典型、病情变化快、精神意识状态难判断,给临床医师评估病情严重性带来极大困难。CNS感染致病菌后,致病菌可刺激脑组织产生一系列细胞因子,这些细胞因子在患儿的免疫反应中发挥重要作用。目前已有多项研究发现脑脊液(cerebrospinal fluid,CSF)中某些细胞因子如白细胞介素(interleukin,IL)6、肿瘤坏死因子α(tumornecrosisfactor-α,TNF-α)等,对PM的早期诊断[1-4]及病情严重程度的评估[5-7]具有重要价值,但这些研究主要针对成年患者或大龄患儿,目前尚缺乏专门针对低龄患儿的研究。本研究通过测定2岁以下低龄PM患儿CSF及血清中TNF-α、IL-6、IL-8和IL-1β的浓度,探讨其在PM患儿早期诊断、病情判断和远期预后评估中的价值。
1 资 料 与 方 法
1.1 一般资料 选择2016年1月—2017年12月在邢台市人民医院儿三科住院的2岁以下PM患儿25例为试验组,其中男性16例,女性9例,月龄中位数2.4个月,四分位数间距5.2个月,发病季节集中在6~8月的有17例(68.0%),脑脊液细菌培养结果阳性3例(肺炎链球菌、脑膜炎奈瑟菌和粪肠球菌各1例)。所有PM患儿均有不同程度的发热、精神差,其中2例伴有头痛,7例伴有呕吐,3例前囟隆起,8例惊厥,6例昏迷,8例伴有硬膜下积液。另选取疑诊为PM、脑脊液常规检查无异常,最终排除颅内感染疾病的患儿18例为对照组,其中男性12例,女性6例,月龄中位数3.8个月,四分位数间距5.9个月,热性惊厥9例、肺炎3例、支气管炎4例、心包炎2例。2组一般资料差异均无统计学意义(P>0.05)。参照Kepa等[5]划分重症脑炎的方法,依据患儿中枢神经系统(central nervous system,CNS)功能障碍的程度将试验组分为重症组(有意识障碍、昏迷、惊厥者)8例和普通组(无惊厥和昏迷)17例。
参照《诸福棠实用儿科学》中诊断PM的标准纳入患儿,排除合并颅内肿瘤、颅内出血、血液病、先天性脑发育异常、免疫系统疾病、除PM以外的感染性脑膜炎及腰穿前应用抗菌药物超过2 d的患儿。所有患儿家长均知情同意且签署知情同意书,此研究经医院伦理委员会批准。
1.2 标本收集与细胞因子检测 患儿在入院12 h内采集静脉血2 mL,通过腰椎穿刺采集脑脊液2 mL。标本在300 g下离心10 min,存放于-80 ℃冰箱中备用。采用酶联免疫吸附(enzyme linked immunosorbent assay,ELISA)法测定血清和CSF中TNF-α、IL-6、IL-8和IL-1β的浓度(ELISA试剂盒均购自北京冬歌博业生物科技有限公司)。
1.3 临床随访和预后评估 随访6个月,观察患儿预后情况。按照儿童格拉斯哥结局评分(Children Glasgow Outcome Scale,CGOS)评定患儿预后[8]。将患儿根据评定结果分为:预后良好组(CGOS评级5级,恢复良好),预后轻度不良组(CGOS评级4级,轻度残疾)和预后不良组(CGOS评级1~3级,包括日常生活无法自理、重症残疾和死亡)。
1.4 统计学方法 应用GraphPad Prism 6.0和SPSS 20.0统计软件分析数据。计数资料比较采用χ2检验,计量资料非正态分布时比较采用Kruskal-Wallis秩和检验。采用受试者工作特征(receiveroperating characteristic,ROC)曲线评估各促炎因子对PM的诊断价值,以及判断患儿病情严重程度的效能。P<0.05为有差异统计学意义。
2 结 果
2.1 3组促炎因子的检出情况 检测标本中TNF-α、IL-1β、IL-6、IL-8的定量限分别为6.0、0.8、5.0和10.0 ng/L。3组脑脊液TNF-α、IL-1β、IL-6、IL-8和血清TNF-α、IL-1β、IL-6差异均有统计学意义(P<0.05或P<0.01)。见表1。
表1 3组促炎因子的检出情况比较
Table 1 Comparinsion of serum proinflammatory factors among three groups (例数,%)
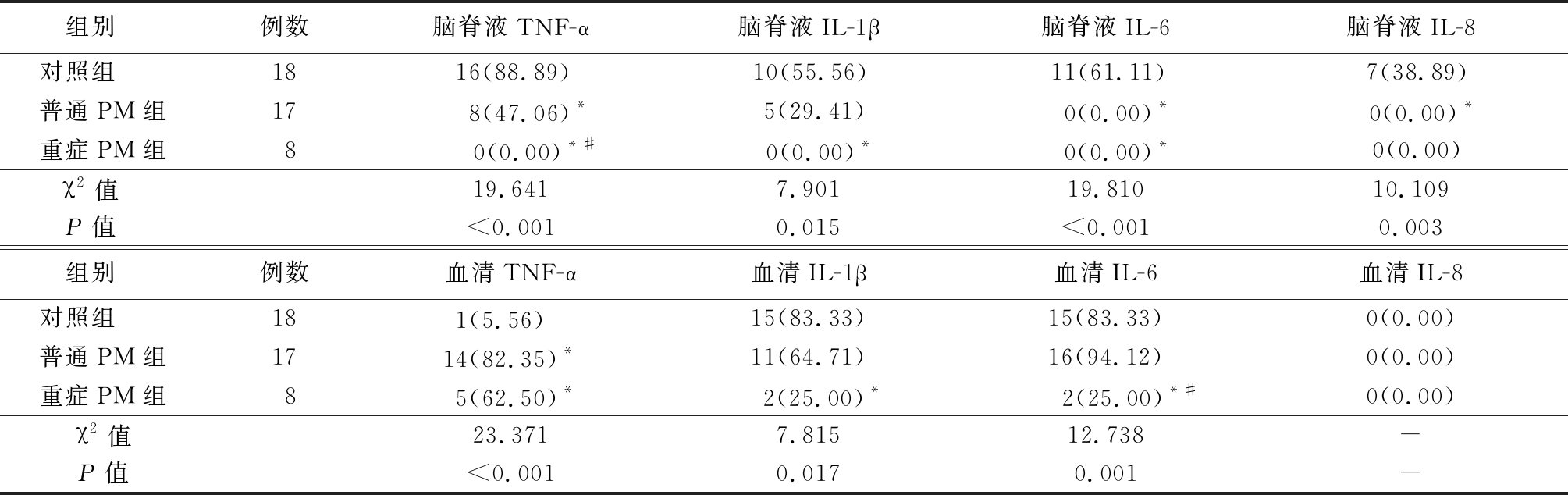
*P值<0.05与对照组比较 #P值<0.05与普通PM组比较(χ2检验)
组别 例数脑脊液TNF-α脑脊液IL-1β脑脊液IL-6脑脊液IL-8对照组 1816(88.89)10(55.56)11(61.11)7(38.89)普通PM组17 8(47.06)*5(29.41) 0(0.00)* 0(0.00)*重症PM组8 0(0.00)*# 0(0.00)* 0(0.00)*0(0.00)χ2值 19.6417.90119.81010.109P值 <0.0010.015<0.0010.003组别 例数血清TNF-α血清IL-1β血清IL-6血清IL-8对照组 181(5.56) 15(83.33)15(83.33)0(0.00)普通PM组1714(82.35)*11(64.71)16(94.12)0(0.00)重症PM组85(62.50)* 2(25.00)* 2(25.00)*#0(0.00)χ2值23.3717.81512.738-P值<0.0010.0170.001-
2.2 促炎因子对PM的诊断价值 CSF和血清中TNF-α、IL-1β、IL-6、IL-8对PM的诊断效能各不相同。在各促炎因子ROC曲线中,以CSF IL-6和CSF IL-8的AUC最大。当CSF IL-6截断值取26.70 ng/L时,约登指数最大,为0.689,此时敏感度和特异度分别为80.0%和88.9%;当CSF IL-8截断值取119.05 ng/L时,约登指数最大,为0.713,此时敏感度和特异度分别为88.0%和83.3%,提示CSF IL-6和CSF IL-8对小儿PM具有较高的诊断价值。见表2。
表2 各促炎因子不同水平阈值诊断PM的ROC曲线参数
Table 2 Pro-inflammatory cytokines levels of different threshold diagnosis of PM ROC curve
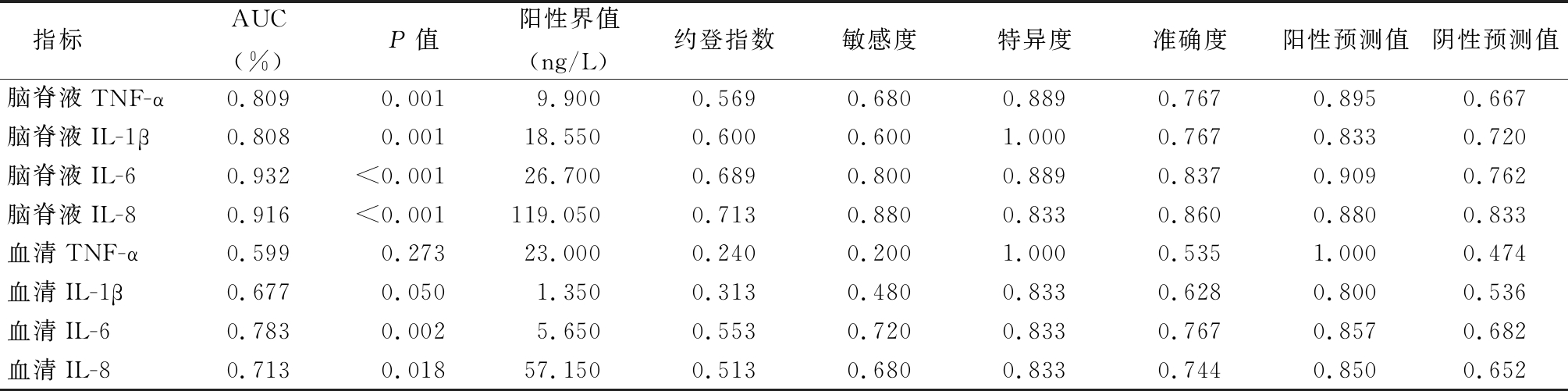
指标AUC(%)P值阳性界值(ng/L)约登指数敏感度特异度准确度阳性预测值阴性预测值脑脊液TNF-α0.8090.0019.9000.5690.6800.8890.767 0.895 0.667 脑脊液IL-1β0.8080.00118.5500.6000.6001.0000.767 0.833 0.720 脑脊液IL-60.932<0.00126.7000.6890.8000.8890.837 0.909 0.762 脑脊液IL-80.916<0.001119.0500.7130.8800.8330.860 0.880 0.833 血清TNF-α0.5990.27323.0000.2400.2001.0000.535 1.000 0.474 血清IL-1β0.6770.0501.3500.3130.4800.8330.628 0.800 0.536 血清IL-60.7830.0025.6500.5530.7200.8330.767 0.857 0.682 血清IL-80.7130.01857.1500.5130.6800.8330.744 0.850 0.652
2.3 促炎因子水平与PM患儿病情轻重程度的关系 普通PM组和重症PM组CSF中TNF-α、IL-1β、IL-6、IL-8水平均较对照组明显升高,差异有统计学意义(P<0.05)。重症PM组CSF中的4种促炎因子水平均显著高于普通PM组(P<0.01),普通PM组促炎因子水平均显著高于对照组(P<0.05),提示PM病情严重程度与CSF中促炎因子水平有一定相关性。见表3。
表3 3组脑脊液促炎因子水平比较
Table 3 Comparison on the CSF levels of serum proinflammatory factors among three groups (M,QR,ng/L)
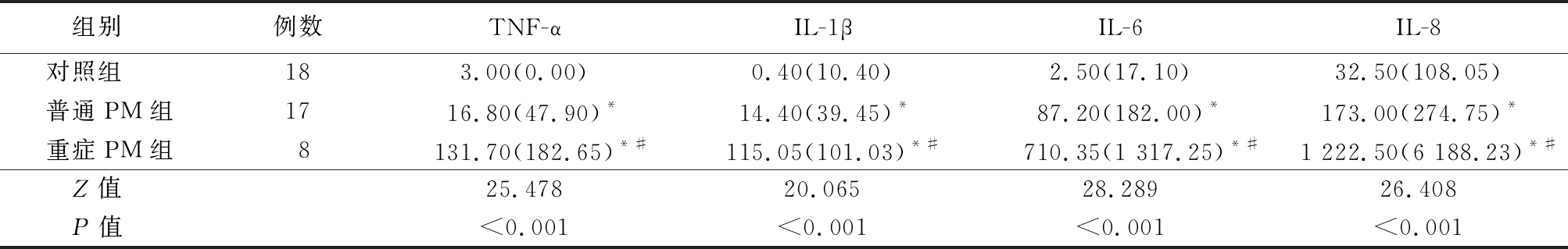
*P值<0.05与对照组比较 #P<0.05与普通PM组比较(秩和检验)
组别 例数TNF-αIL-1βIL-6IL-8对照组 183.00(0.00) 0.40(10.40)2.50(17.10)32.50(108.05)普通PM组1716.80(47.90)*14.40(39.45)*87.20(182.00)*173.00(274.75)*重症PM组8131.70(182.65)*#115.05(101.03)*#710.35(1 317.25)*#1 222.50(6 188.23)*#Z值 25.47820.06528.28926.408P值 <0.001<0.001<0.001<0.001
表4 3组血清促炎因子水平比较
Table 4 Comparison on the serum levels of serum proinflammatory factors among three groups (M,QR,ng/L)
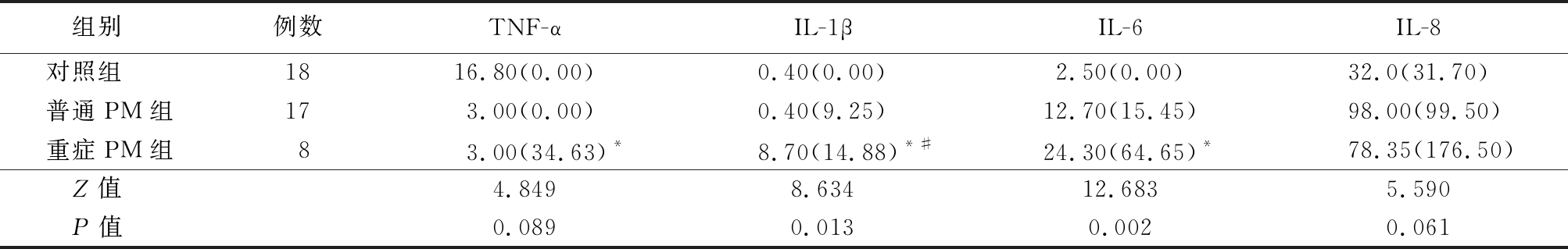
*P值<0.05与对照组比较 #P值<0.05与普通PM组比较(秩和检验)
组别 例数TNF-αIL-1βIL-6IL-8对照组 1816.80(0.00)0.40(0.00)2.50(0.00)32.0(31.70)普通PM组173.00(0.00)0.40(9.25)12.70(15.45)98.00(99.50)重症PM组83.00(34.63)*8.70(14.88)*#24.30(64.65)*78.35(176.50)Z值 4.8498.63412.6835.590P值 0.0890.0130.0020.061
2.4 促炎因子对PM病情轻重区分的效能 在所有区分重症PM与普通PM的ROC曲线中,CSF中TNF-α的AUC最大。当CSF TNF-α截断值为70.4 ng/L时,约登指数最大,为0.941,此时区分普通PM与重症PM的敏感度为100%、特异度为94.1%。见图1,表5。
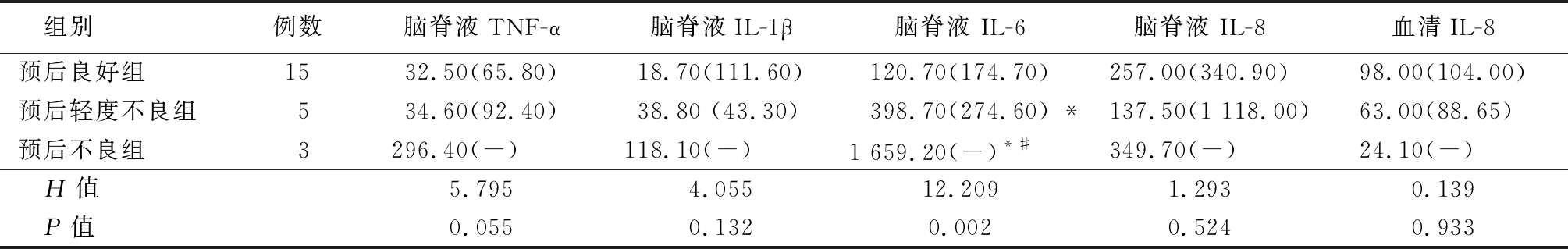
2.5 CSF和血清中4种促炎因子对PM预后的评估价值 患儿出院6个月后,共有23例PM患儿完成随访,预后良好组15例,预后轻度不良组5例,预后不良组3例。因患儿血清中TNF-α、IL-6和IL-1β的浓度低于定量限的标本数量较多,故未对这3个检测指标进行统计学分析。对另外5个检测指标进行秩和检验,结果显示,预后轻度不良组脑脊液IL-6水平明显高于预后良好组,预后不良组脑脊液IL-6水平高于预后良好组和预后轻度不良组,差异有统计学意义(P<0.05)。见表6。
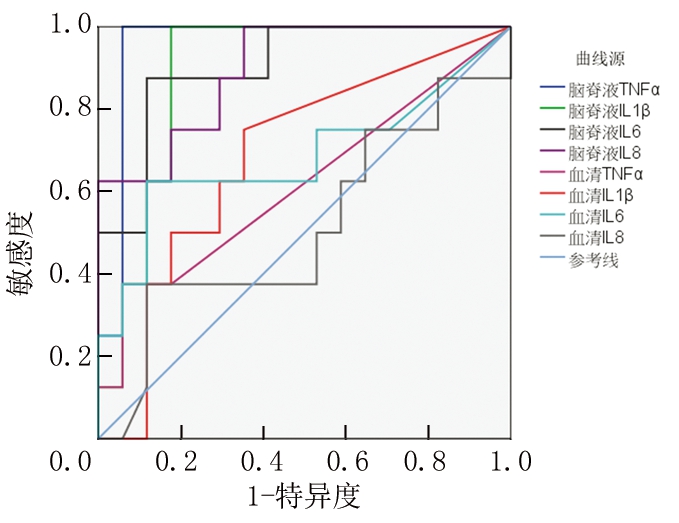
图1 CSF和血清中TNF-α、IL-1β、IL-6、IL-8区分PM病情轻重的ROC曲线
Figure 1 ROC curve of levels of TNF-α,IL-1β,IL-6 and IL-8 in CSF and serum distinguishing disease severity of PM
表5 各促炎因子评估PM病情轻重的ROC曲线参数
Table 5 ROC curve parameters of pro-inflammatory cytokines in evaluation of disease severity of pediatric PM
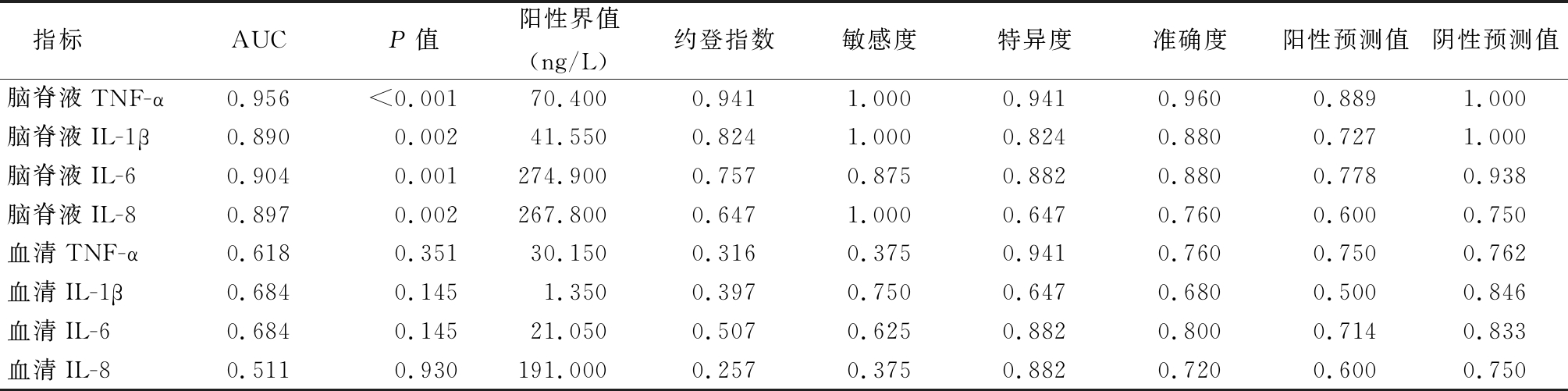
指标AUCP值阳性界值(ng/L)约登指数敏感度特异度准确度阳性预测值阴性预测值脑脊液TNF-α0.956<0.00170.4000.9411.0000.9410.960 0.889 1.000 脑脊液IL-1β0.8900.00241.5500.8241.0000.8240.880 0.727 1.000 脑脊液IL-60.9040.001274.9000.7570.8750.8820.880 0.778 0.938 脑脊液IL-80.8970.002267.8000.6471.0000.6470.760 0.600 0.750 血清TNF-α0.6180.35130.1500.3160.3750.9410.760 0.750 0.762 血清IL-1β0.6840.1451.3500.3970.7500.6470.680 0.500 0.846 血清IL-60.6840.14521.0500.5070.6250.8820.800 0.714 0.833 血清IL-80.5110.930191.0000.2570.3750.8820.720 0.600 0.750
表6 不同预后PM患儿的各项指标比较
Table 6 Comparison of related measure indexes levels in different prognosis children with purulent meningitis (M,QR,ng/L)
*P值<0.05与预后良好组比较 #P值<0.05与预后轻度不良组比较 (秩和检验)
组别例数脑脊液TNF-α脑脊液IL-1β脑脊液 IL-6脑脊液 IL-8血清IL-8预后良好组1532.50(65.80)18.70(111.60)120.70(174.70)257.00(340.90)98.00(104.00)预后轻度不良组534.60(92.40)38.80 (43.30)398.70(274.60) *137.50(1 118.00)63.00(88.65)预后不良组3296.40(-)118.10(-)1 659.20(-)*#349.70(-)24.10(-) H值5.7954.05512.2091.2930.139 P值0.0550.1320.0020.5240.933
3 讨 论
低龄PM患儿的临床表现趋于不典型,本研究中,PM患儿年龄小(中位年龄2.4个月),头痛、脑膜刺激征、前囟隆起等临床表现发生率仅为8%~12%。脑脊液细菌培养作为诊断PM的金标准[9],其培养阳性率太低(本研究仅12%)。早期诊断和判断病情是合理治疗、改善预后的关键,因此有必要寻找能早期诊断PM和评估病情的可靠标志物。多项研究表明,CSF中TNF-α、IL-6、IL-8等促炎因子对PM具有较高的诊断价值[1-4,10-13]。Srinivasan等[1]分析了608名婴儿CSF中TNF-α、IL-1、IL-6、IL-8、IL-10和IL-12诊断PM的效能,认为IL-6和IL-10在诊断PM方面具更高的准确性。Dano等[2]观测65例15岁以下PM患儿CSF中IL-6的水平,发现PM组水平显著高于对照组,其诊断脑膜炎的ROC曲线AUC达0.94,对诊断PM具有良好的效能。本研究结果显示,PM组CSF中TNF-α、IL-8、IL-1β、IL-6的水平均显著高于对照组的水平;CSF中IL-6和IL-8独立诊断PM的ROC曲线下面积均大于0.90,两者在最佳截断值时的敏感度和特异度均在80%以上,说明CSF IL-6和CSF IL-8对PM均具有较高的诊断效能,这与一项Meta分析研究报道结果基本相符[3]。
病原菌入侵CNS后,刺激免疫细胞向感染灶浸润聚集,活化的巨噬细胞释放TNF-α、IL-1、IL-6等细胞因子。TNF-α是重要的早期促炎细胞因子,在CNS感染中发挥先导作用,可进一步诱导IL-6、IL-8、IL-1β等促炎因子的合成和释放[14],增强炎症细胞的黏附作用,提高血脑屏障通透性[15]。IL-6是一种促炎细胞因子,能激活基质蛋白金属酶,破坏血脑屏障[16],还可刺激细胞产生活性氧等毒性物质。IL-8对中性粒细胞具有趋化性,能促进局部炎症。一般来说,促炎因子水平越高,表明炎症反应程度越重,炎症对组织的损伤越大。有研究表明PM患者CSF TNF-α水平与血脑屏障损伤程度和病情严重程度具有显著相关性[6-7]。Kepa等[5]比较了轻度、中度及重度成年PM患者的CSF IL-6水平,结果显示CSF IL-6水平与成年PM患者病情严重程度具有相关性,可用于评估患者病情的严重程度。与上述文献结果基本一致,本研究结果显示,PM组患儿CSF中TNF-α、IL-1β、IL-6、IL-8水平均较对照组明显升高,且重症PM组促炎因子水平显著高于普通PM组,普通PM组显著高于对照组水平,提示CSF中TNF-α、IL-1β、IL-6、IL-8等促炎因子水平与PM病情严重程度密切关系。区分PM病情轻重的ROC分析显示,CSF中TNF-α的AUC最大(0.956),IL-6次之(0.904),IL-8(0.897)和IL-1β(0.890)再次之,而血清中TNF-α、IL-1β、IL-6、IL-8的AUC均不足0.70,说明血清样品中的促炎因子判断PM病情轻重的效能均不及脑脊液样品,CSF TNF-α和CSF IL-6可作为判断低龄PM患儿病情严重性的敏感指标。
值得说明的是,对照组和PM组患儿CSF和血清中TNF-α的检出率均较低,对照组CSF和血清中检出率分别仅有11.1%和5.6%,PM组分别为68%和24%。动物实验显示,肺炎链球菌性脑膜炎大鼠感染细菌6 h时CSF中TNF-α水平开始升高,但在24 h后多数CSF标本已检测不到TNF-α[17]。PM患者接受抗菌药物治疗24 h后就不易检测出CSF中TNF-α[6]。而IL-1β、IL-6、IL-8等促炎因子的半衰期较长[18],动物感染96 h后这些炎症因子仍有较高浓度[17,19]。可见CSF细胞因子的水平与发病时间有密切关系。由于本研究不可能准确获知每例患儿患病的确切时间,但纳入本研究的均是出现症状之后48 h以内的患儿。部分患儿采集标本时已经错过了TNF-α的高峰期,这可能是入组患儿TNF-α检出率低的原因。虽然TNF-α的检出率不高,但TNF-α的检出结果显示,重症PM组患儿CSF中TNF-α的水平显著高于普通PM组(P<0.001),说明CSF中高水平的TNF-α往往预示着患儿PM病情严重。
Takahashi等[4]和V zquez等[19]观察PM成年患者CSF中IL-6水平与预后的相关性,结果显示CSF中IL-6水平越高,近期预后越差。Kepa等[5]研究证实,在轻、中、重症PM成年患者CSF中,IL-6水平与蛛网膜下腔的炎症反应强度及脑损伤预后密切相关,CSF中高水平的IL-6意味着脑损伤严重、临床结局恶化。与上述研究结果一致,本研究显示,预后轻度不良组脑脊液IL-6水平明显高于预后良好组,预后不良组脑脊液IL-6水平显著高于预后轻度不良组,表明PM患儿脑脊液中IL-6水平越高,患儿的远期预后就越差。IL-6是中性粒细胞向CNS迁移的强效诱导剂,一方面可激活中性粒细胞胞内酶,增强对病原体的杀伤力,另一方面也可对组织造成炎性损伤。但本研究与上述文献研究的样本量均较小,且未对危险因素进行分层,得出的结论可能存在偏倚。因此尚需开展大样本的研究,以确认IL-6等炎性因子预测PM预后的准确性。
zquez等[19]观察PM成年患者CSF中IL-6水平与预后的相关性,结果显示CSF中IL-6水平越高,近期预后越差。Kepa等[5]研究证实,在轻、中、重症PM成年患者CSF中,IL-6水平与蛛网膜下腔的炎症反应强度及脑损伤预后密切相关,CSF中高水平的IL-6意味着脑损伤严重、临床结局恶化。与上述研究结果一致,本研究显示,预后轻度不良组脑脊液IL-6水平明显高于预后良好组,预后不良组脑脊液IL-6水平显著高于预后轻度不良组,表明PM患儿脑脊液中IL-6水平越高,患儿的远期预后就越差。IL-6是中性粒细胞向CNS迁移的强效诱导剂,一方面可激活中性粒细胞胞内酶,增强对病原体的杀伤力,另一方面也可对组织造成炎性损伤。但本研究与上述文献研究的样本量均较小,且未对危险因素进行分层,得出的结论可能存在偏倚。因此尚需开展大样本的研究,以确认IL-6等炎性因子预测PM预后的准确性。
综上所述,低龄PM患儿CNS内产生大量促炎因子,脑脊液中IL-6和IL-8可作为早期诊断PM的辅助指标。脑脊液中促炎因子水平越高,预示着病情越严重,其中,TNF-α和IL-6对判断PM患儿病情轻重具有更高的敏感度和特异度,本研究所得的TNF-α和IL-6判断病情轻重的最佳截断值可为医师判断病情轻重提供参考。另外,CSF中IL-6水平可能有助于评估PM远期预后,但其确切价值尚需进一步开展大样本的临床研究以证实。
[1] Srinivasan L,Kilpatrick L,Shah SS,et al. Cerebrospinal fluid cytokines in the diagnosis of bacterial meningitis in infants[J]. Pediatr Res,2016,80(4):566-572.
[2] Dano ID,Sadou H,Issaka B,et al. Measurement of interleukin-6 in cerebrospinal fluid for the diagnosis of bacterial meningitis[J]. Pak J Biol Sci,2016,19(4):185-190.
[3] Yao R,Cao Y,Chen Y,et al. Diagnostic performance of interleukin-6 and interleukin-8 for bacterial meningitis:a meta-analysis[J]. Int J Clin Exp Med,2015,8(5):7059-7068.
[4] Takahashi W,Nakada TA,Abe R,et al. Usefulness of interleukin 6 levels in the cerebrospinal fluid for the diagnosis of bacterial meningitis[J]. J Crit Care,2014,29(4):693.e1-e6.
[5] Kepa L,Oczko-Grzesik B,Boro -Kaczmarska A. Cerebrospinal fluid interleukin-6 concentration in patients with purulent,bacterial meningitis-own observations[J]. Przegl Epidemiol,2014,68(4):645-649.
-Kaczmarska A. Cerebrospinal fluid interleukin-6 concentration in patients with purulent,bacterial meningitis-own observations[J]. Przegl Epidemiol,2014,68(4):645-649.
[6] Sharief MK,Ciardi M,Thompson EJ. Blood-brain barrier damage in patients with bacterial meningitis:association with tumor necrosis factor-alpha but not interleukin-1 beta[J]. J Infect Dis,1992,166(2):350-358.
[7] Arditi M,Manogue KR,Caplan M,et al. Cerebrospinal fluid cachectin tumor necrosis factor alpha and platelet activating factor concentrations and severity of bacterial meningitis in children[J]. J Infect Dis,1990,162(1):139-147.
[8] Lax Pericall MT,Taylor E. Family function and its relationship to injury severity and psychiatric outcome in children with acquired brain injury:a systematized review [J].Dev Med Child Neurol,2014,56(1):19-30.
[9] García-Hern ndez P,Prieto B,Martínez-Morillo E,et al. Interleukin-6 in cerebrospinal fluid as a biomarker of acute meningitis[J]. Ann Clin Biochem,2016,53(Pt 1):155-163.
ndez P,Prieto B,Martínez-Morillo E,et al. Interleukin-6 in cerebrospinal fluid as a biomarker of acute meningitis[J]. Ann Clin Biochem,2016,53(Pt 1):155-163.
[10] 雷鸿雁. TNF-α、ILs等炎性因子检测在脑膜炎诊断中的价值分析[J].中国医药指南,2018,16(2):156-157.
[11] Zhang G,Yang C,Kang X,et al. The combination of cerebrospinal fluid procalcitonin,lactate,interleukin-8 and interleukin-10 concentrations for the diagnosis of postneurosurgical bacterial meningitis:A prospective study[J]. Ann Clin Biochem,2019,56(1):133-140.
[12] Mazankova LN,Milovanova OA,Moiseenkova DA,et al. Neurological presentations of bacterial meningitis in children:current possibilities of diagnosis and treatment[J]. Zh Nevrol Psikhiatr Im S S Korsakova,2016,116(6):4-9.
[13] Ye Q,Shao W,Shang S,et al. Clinical value of assessing cytokine levels for the differential diagnosis of bacterial meningitis in a pediatric population[J]. Medicine(Baltimore),2016,95(13):e3222.
[14] 温雅,董立鹏,赵景茹,等.旋覆花内酯通过抑制炎症反应对缺血脑组织发挥保护作用[J].河北医科大学学报,2016,37(5):497-500,505.
[15] Al-Obaidi MMJ,Desa MNM. Mechanisms of blood brain barrier disruption by different types of bacteria,and bacterial-host interactions facilitate the bacterial pathogen invading the brain[J]. Cell Mol Neurobiol,2018,38(7):1349-1368.
[16] Rodriguez-Smith J,Lin YC,Tsai WL,et al. CSF cytokines correlate with aseptic meningitis and blood brain barrier function in Neonatal-Onset Multisystem Inflammatory Disease(NOMID)[J]. Arthritis Rheumatol,2017,69(6):1325-1336.
[17] Barichello T,dos Santos I,Savi GD,et al. TNF-alpha,IL-1beta,IL-6,and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae[J]. J Neuroimmunol,2010,221(1/2):42-45.
[18] Barichello T,Generoso JS,Silvestre C,et al. Circulating concentrations,cerebral output of the CINC-1 and blood-brain barrier disruption in Wistar rats after pneumococcal meningitis induction[J]. Eur J Clin Microbiol Infect Dis,2012,31(8):2005-2009.
[19] V zquez JA,Adducci Mdel C,Coll C,et al. Acute meningitis prognosis using cerebrospinal fluid interleukin-6 levels[J]. J Emerg Med,2012,43(2):322-327.
zquez JA,Adducci Mdel C,Coll C,et al. Acute meningitis prognosis using cerebrospinal fluid interleukin-6 levels[J]. J Emerg Med,2012,43(2):322-327.