肝转移瘤(liver metastases,LM)是肝脏常见的恶性肿瘤,是影响肿瘤患者生存期的重要影响因素[1-2]。临床上对于LM的治疗包括手术切除、化疗、放疗、动脉灌注化疗/栓塞(hepatic artery infusion chemotherapy/transcatheter arterial chemoembolization,HAI/TACE)治疗及靶向治疗等[3],而能手术切除的LM患者仅为10%~20%[4],多数采用非手术治疗。目前研究化疗及靶向治疗等对LM疗效的临床及实验研究多使用小鼠或裸鼠LM模型[5-6],而研究HAI/TACE治疗LM疗效的动物模型少见报道。经脾种植VX2瘤株形成兔LM模型已成为一种技术成熟的动物模型[7],为进一步明确经脾种植VX2瘤株兔LM模型数字减影血管造影(digital subtraction angiography,DSA)及肝动脉锥形束强化CT表现,能否满足临床上观察HAI/TACE治疗的相应条件,能否成为研究HAI/TACE治疗LM的理想动物模型,对于临床上研究HAI/TACE治疗LM疗效具有重要意义。
1 材料与方法
1.1 建立VX2瘤株兔LM模型 选取新西兰大白兔40只,配置新鲜的VX2瘤株悬液。悬液配置成功后,沿腹部切口,游离脾脏并用1 mL注射器将VX2瘤株悬液注射入脾脏内。种植后15 d行CT增强扫描,观察肝内是否存在LM(增强扫描显示LM动脉期强化特点为转移瘤周边呈环形强化,中心区域未见强化)[7]。对增强CT确认肝内成功转移的兔于第2天行DSA及肝动脉锥形束强化CT。
1.2 VX2瘤株兔LM模型股动脉插管 沿右侧腹股沟下2 cm触及股动脉,沿股动脉搏动方向逐层分离兔大腿皮层及肌层,股动脉暴露后钝性分离股动脉,将股动脉与股静脉、股神经充分分离,股动脉分离长度1~2 cm。分离成功后将股动脉近端缝合线游离待用,股动脉远端用缝合线结扎,并用血管夹夹闭股动脉远端。待股动脉明显增粗后,轻提远端缝合线,使股动脉稍上提,用5 F穿刺针穿刺股动脉,穿刺针尾端冒血后将穿刺套管前送,直至穿刺套管充分进入股动脉内。将游离股动脉近端用血管夹固定套管,连接“Y”型止血阀,并注射肝素防止血栓形成(肝素用量按10 mg/kg)。
1.3 腹腔动脉及肝动脉DSA 通过“Y”阀,将2.2 F Stride微导管和微导丝置入动脉内,在微导丝引导下将微导管置于平兔12胸椎与腰1椎体处,行腹部背大动脉造影(注射速度2 mL/s,总量8 mL),观察腹腔动脉及肝动脉开口走行。根据肝动脉开口走行将微导管插入肝动脉内。微导管选入肝动脉内后,行肝动脉造影(注射速度1.0 mL/s,总量4 mL),观察肝动脉走行分布及LM的染色情况,并行肝动脉锥形束CT增强扫描,观察LM强化情况。
1.4 间接门静脉DSA 在肝动脉造影及锥形束CT强化结束后,将微导管选入胃脾动脉干内行间接门静脉造影(注射速度1.5 mL/s,总量8 mL),观察LM间接门静脉造影下的染色情况。
1.5 LM的HE染色 在DSA成功后,将兔处死,对LM进行分离、脱水、固定及HE染色,镜下观察组织学表现。
1.6 图像分析 由2名副主任医师以上专家评价VX2瘤株兔LM的DSA、肝动脉锥形束强化CT、间接门静脉造影表现及检查出的肿瘤染色情况;比较DSA、锥形束强化CT、间接门静脉造影表现与腹部增强CT强化及HE病理表现。
2 结 果
VX2瘤株兔LM模型种植成功37只,成功率为92.5%(37/40)。因麻醉死亡1只,未能种植成功1只,行CT增强扫描时死亡1只。37只VX2瘤株兔LM模型的CT增强扫描共发现LM病灶44个。37只种植成功的VX2瘤株兔LM模型行股动脉穿刺插管,1次穿刺插管成功32只,穿刺成功率为86.5%(32/37),2次穿刺成功5只。腹部背大动脉造影发现兔腹腔动脉开口位于胸12椎体中部水平,与人腹腔干开口位置大致一致。但是兔腹腔动脉较短,胃肝动脉较长。胃肝动脉分出肝动脉。
2.1 VX2瘤株兔LM的DSA及肝动脉锥形束CT强化表现 37只VX2瘤株兔LM模型经DSA发现LM病灶57个,其中中等血供病灶38个、乏血供病灶19个。DSA造影表现:肝内动脉走行迂曲,LM的肿瘤供血支紊乱、不规则,染色呈类圆形,中心区域染色较边缘染色稍浅淡,实质期LM染色进一步加重,但是染色程度低于正常肝实质。锥形束强化CT表现:多发大小不等LM,强化特点为转移瘤周边呈环形强化,中心区域未见强化。与增强CT对比分析发现,DSA造影的染色情况、锥形束强化CT强化表现均于增强CT一致。见图1~5。
2.2 间接门静脉造影DSA表现 门静脉主干粗大,各叶分支门静脉显示清晰,门静脉分支逐级分支,走行规则,肝内未见明显肿瘤染色,肝内染色均匀一致。
2.3 HE染色镜下观察 大体解剖兔VX2瘤株LM共发现及制作79个蜡块,LM的DSA染色部分、肝动脉锥形束CT肿瘤强化部位及程度与腹部增强CT的LM强化表现一致,为LM周边强化,对应于HE镜下观察肿瘤外周存在活性的肿瘤细胞、炎性细胞及结缔组织[7];LM的DSA中心染色浅淡、肝动脉锥形束CT未强化部位与腹部增强CT的LM未强化表现一致[7],对应HE镜下肿瘤中心分布嗜碱性红染坏死肿瘤细胞。见图6~7。
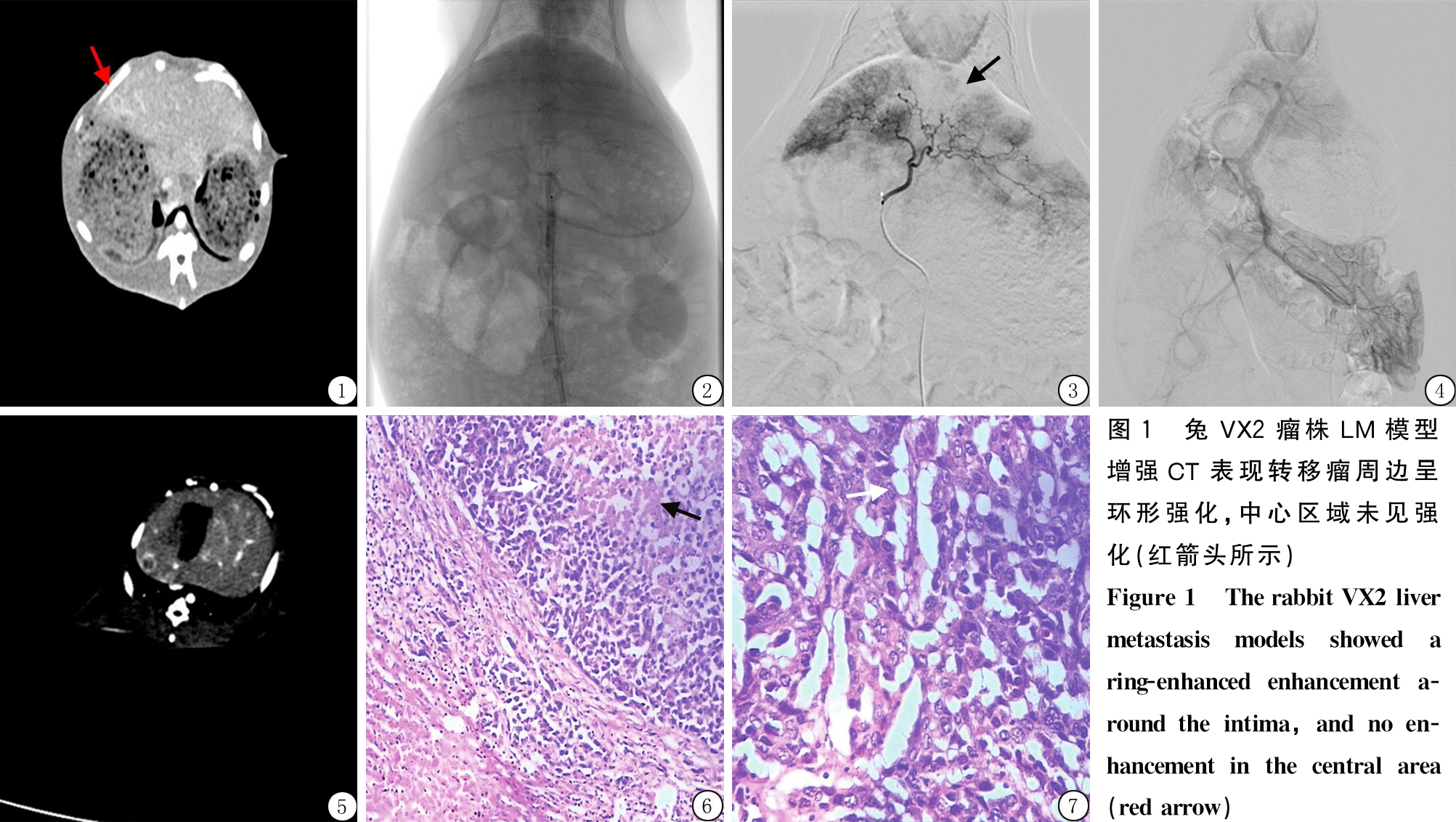
图1 兔VX2瘤株LM模型增强CT表现转移瘤周边呈环形强化,中心区域未见强化(红箭头所示)Figure1 TherabbitVX2livermetastasismodelsshowedaring-enhancedenhancementa-roundtheintima,andnoen-hancementinthecentralarea(redarrow)
图2 背大动脉造影示腹腔动脉开口于胸12椎体水平
Figure 2 Dorsal aortic angiography revealed the opening of the abdominal artery at the level of the thoracic 12 vertebra
图3 兔VX2瘤株LM模型DSA表现为供血支紊乱、不规则,动脉中晚期肿瘤染色,染色浅淡(黑箭头所示)
Figure 3 The DSA of the rabbit VX2 liver metastasis model revealed disordered and irregular blood supply branches,and the staining of tumor was light in middle and late arteries(black arrow)
图4 兔VX2瘤株LM模型间接门静脉造影DSA表现为门静脉主干粗大,各叶分支门静脉显示清晰,肝内未见明显肿瘤染色
Figure 4 Indirect portal venography DSA of rabbit VX2 liver metastasis model revealed that the main trunk of the portal vein was wide, the portal vein of each branch was clear, and no obvious tumor staining was seen in the liver
图5 兔VX2瘤株LM模型锥形束CT增强扫描示转移瘤周边呈环形强化,中心区域未见强化,与腹部增强CT表现一致
Figure 5 The cone-beam CT enhanced scan of rabbit VX2 liver metastasis model showed a ring-enhanced enhancement around the intima, and no enhancement in the central area, which was consistent with the performance of abdominal enhanced CT
图6 低倍镜下观察LM外周存在活性的肿瘤细胞(白色箭头)、炎性细胞及结缔组织,中心分布嗜碱性红染坏死肿瘤细胞(黑色箭头)(HE ×100)
Figure 6 The low-power microscope was used to observe active tumor cells(white arrows), inflammatory cells and connective tissue on the periphery of liver metastases, and basophilic red stained necrotic tumor cells(black arrows) in the center(HE ×100)
图7 高倍镜下观察LM活性肿瘤细胞(白色箭头)形态欠规则,细胞核大而深染,胞质量少(HE ×400)
Figure 7 LM active tumor cells(white arrows) were not regular in morphology, with large and deep stained nuclei and low cell mass under high-power microscope(HE ×400)
3 讨 论
肝脏是消化道恶性肿瘤最常见的转移器官,如果不进行有效治疗,其中位生存期仅为6.9个月[1-2,8]。在LM的各种治疗方法中,非手术治疗是LM最常用的治疗方法,化疗、靶向治疗及免疫治疗等方法对于LM的疗效均在动物实验及临床实验中取得了证实[6,9-10]。随着介入技术及栓塞剂的发展,尤其是载药微球的临床应用,HAI/TACE在LM治疗中的应用越来越广泛[11-13]。但是HAI/TACE对LM的疗效仍存在着争议[14-15]。因而,建立一种符合LM生物学特性,可以进行HAI/TACE治疗,并且能观察HAI/TACE治疗疗效的动物模型对于临床上进一步研究具有重要意义。
VX2瘤株肿瘤模型是一种成熟的动物模型,已运用到多种恶性肿瘤的实验及临床研究中,尤其是肝癌模型中[16-17]。一种理想的动物模型应该与人类的生物学特性一致。前期研究证实,经脾种植VX2瘤株形成LM模型接近人LM的生物学特性,是一种理想地研究LM的动物模型[7]。但是其能否成为研究HAI/TACE治疗LM的理想模型仍需进一步证实。本研究通过研究经脾种植VX2瘤株兔LM模型的DSA表现,观察肝内血管走形及LM染色情况,并观察LM病理结构,分析其能否成为研究HAI/TACE治疗LM的理想模型。
人LM的DSA表现有3种:富血供、中等血供及乏血供[18]。本研究成功建立VX2瘤株兔LM模型37只,发现LM病灶57个,其中中等血供病灶38个、乏血供病灶19个。LM的DSA表现为肝内动脉走行迂曲,LM供血支紊乱、不规则,这与人LM的中等血供及乏血供的血管走行表现相似;并且VX2瘤株兔LM在动脉中晚期呈类圆形肿瘤染色,较大LM可观察到中心区域染色较边缘染色稍浅淡。实质期LM染色进一步加重,但是低于正常肝实质染色,这与人中等血供及乏血供的LM染色情况大致一致。肝动脉锥形束强化CT表现可见肝内LM强化特点为转移瘤周边呈环形强化,中心区域未见强化,VX2瘤株兔LM的肝动脉锥形束强化CT表现与DSA造影前CT表现一致,提示经脾种植VX2瘤株形成的兔LM模型适合于临床上影像学表现为环形强化的人LM的研究,与前期研究一致[7]。VX2瘤株兔LM模型的大体病理镜下表现为活性的肿瘤细胞、炎性细胞及结缔组织分布于肿瘤外周,与DSA染色部分及肝动脉锥形束CT肿瘤强化部位情况一致,而肿瘤中心分布嗜碱性红染坏死肿瘤细胞,与DSA中心染色浅淡及锥形束CT未强化部位一致。
另外,进行了VX2瘤株兔LM模型间接门静脉造影,造影表现为门静脉主干粗大,各叶分支门静脉显示清晰,门静脉分支逐级分支,走行规则,肝内未见明显肿瘤染色,肝内染色均匀一致。这提示该肿瘤模型能成功行间接门静脉造影,能对临床上研究LM血供等研究提供合适的LM模型。经脾种植VX2瘤株形成的兔LM模型符合临床上影像学表现为环形强化,DSA为中等血供或乏血供的LM,能够为临床提供理想的研究肝转移的HAI/TACE治疗疗效及其他介入实验研究的模型。
笔者将经脾种植兔VX2瘤株LM模型行DSA经验和注意点总结如下:①行兔股动脉插管时应在直视下进行穿刺,并结扎股动脉远端,待股动脉明显增粗后进行穿刺,成功率较高,本研究1次穿刺成功率为86.5%,2次成功率为100%;②行股动脉穿刺时选用穿刺针为5 F穿刺针,穿刺成功率及穿刺后操作更佳,使用>5 F型号穿刺针容易造成股动脉穿刺不成功,而<5 F穿刺针穿刺后微导管无法顺利进入穿刺鞘;③穿刺成功后应用穿刺针自带针鞘替代为导管鞘,使用导管鞘容易造成人为性股动脉撕裂或髂动脉撕裂,而使用针鞘后可完全避免上述情况发生;④选用2.2 F Stride微导管和微导丝对肝动脉进行插管,既能顺利通过针鞘又能对肝动脉进行超选择,>2.2 F的微导管无法顺利通过5 F针鞘,<2.2 F的微导管再行DSA造影时显影较2.2 F导管差;⑤在胸12椎体中部水平进行常规背大动脉造影,能够清晰显示兔腹腔动脉走形及开口方向,进行肝动脉超选择插管,能有效地减少介入术中时间;⑥2.2 F Stride微导管能超选择至兔肝动脉二级分支水平,如需研究精准TACE治疗或载药微球TACE(drug-eluting beads TACE,DEB-TACE)治疗LM的疗效时,建议更换更细微导管,减轻栓塞剂对正常肝组织损伤。
本文研究不足之处在于无法完全消除兔呼吸运动对DSA及肝动脉锥形束强化CT图像质量的影响,部分LM无法清晰观察染色情况,对于临床上行HAI /TACE治疗时影响较大。课题组将进一步研究如何减轻呼吸运动对该模型的影响,进一步完善该模型对临床TACE治疗的需求。
[1] 秦新裕,许剑民,任黎,等.2018版《中国结直肠癌肝转移诊断和综合治疗指南》解读[J].临床外科杂志,2019,27(1):9-13.
[2] Chen W,Sun K,Zheng R,et al. Cancer incidence and mortality in China,2014[J]. Chin J Cancer Res,2018,30(1):1-12.
[3] 冀晓楠,隋红.结直肠癌肝转移新辅助治疗研究进展[J/CD].中华结直肠疾病电子杂志,2018,7(4):368-372.
[4] 刘虎,杨家和.结直肠癌肝转移的转化治疗研究新进展[J].肝胆外科杂志,2018,26(6):472-475.
[5] 孙钰,严卿莹,阮善明,等.转移性结直肠癌动物模型的研究进展[J].肿瘤学杂志,2015,21(4):335-339.
[6] 江涛,吴兆映,史沛聪,等.端粒酶抑制剂1对裸鼠结直肠癌转移的影响[J].中国肿瘤外科杂志,2018,10(1):13-18.
[7] 吴勇超,李智岗,史博,等. 经脾种植VX2瘤株悬液建立兔肝转移瘤模型[J].介入放射学杂志,2019,28(9):861-864.
[8] 中国医疗保健国际交流促进会,结直肠癌肝转移分会.中国医促会结直肠癌肝转移分会结直肠癌肝转移MDT诊治共识(讨论版)[J/CD].肝癌电子杂志,2017,4(2):1-12.
[9] Primrose J,Falk S,Finchjones M,et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis:the New EPOC randomised controlled trial[J]. Lancet Oncology,2014,15(6):601-611.
[10] Goldberg RM,Sargent DJ,Morton RF,et al. A randomized controlled trigal of fluorouracil plus leucvorin,irinotecan,and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer[J]. J Clin Oncol,2004,22(1):23-30.
[11] 吴建兵.肝转移癌经动脉介入治疗进展[J/CD].肝癌电子杂志,2018,5(1):30-33.
[12] 徐新建,滕飞,杜洪涛.伊立替康载药微球治疗结肠癌肝转移瘤的系统评价[J/CD].中华介入放射学电子杂志,2017,5(4):273-281.
[13] 茅锐,张业繁,陈晓,等.肝动脉灌注化疗在结直肠癌肝转移术后辅助治疗中的应用[J/CD].肝癌电子杂志,2017,4(2):21-23.
[14] 王凯,王丽英,刘国兴,等.持续经动脉灌注联合栓塞治疗胃癌肝转移疗效评价[J].介入放射学杂志,2018,27(12):1160-1162.
[15] Liu DM,Thakor AS,Baerlocher M,et al. A review of conventional and drug-eluting chemoembolization in the treatment of colorectal liver metastases:principles and proof[J]. Future Oncol,2015,11(9):1421-1428.
[16] 李浩,段旭华,韩新巍,等.载药微球三氧化二砷治疗兔VX2肝肿瘤药代动力学研究[J].中华放射学杂志,2019,53(7):615-620.
[17] 冯国栋,余辉,冯建防,等.经胃左动脉注入VX2瘤粒建立兔胃癌模型[J].介入放射学杂志,2018,27(6):544-548.
[18] 冯晓波,张彦舫,陈宪,等.肝转移性肿瘤DSA表现分析[J].临床放射学杂志,2000,19(10):637-639.