随着传统医学模型向生物-心理-社会医学模式逐渐转变,证实慢性应激与肿瘤的发生发展密切相关,给予肿瘤患者心理治疗、社会支持、针对慢性应激的药物治疗均可以改善患者的预后[1]。基础研究证实,慢性应激通过应激激素(肾上腺素、去甲肾上腺素、糖皮质激素等)促进肿瘤的发生发展,而免疫因素在慢性应激促肿瘤进展中具有重要作用[2-3]。高表达趋化因子受体2(chemokine receptor 2,CCR2)的单核细胞趋化至肿瘤组织将分化为肿瘤相关巨噬细胞(tumor-associated macrophages,TAM),而TAM通过多种途径促进肿瘤进展[4]。有文献报道慢性应激通过增加体内CCR2+单核细胞的比例进而促进肿瘤进展[5]。脾脏在肿瘤发生发展中具有重要作用。临床研究表明,外伤脾切除会增加肿瘤发生的风险,而切除病理脾脏(如门静脉高压症脾功能亢进的脾脏)能降低肝癌的发生风险[6-7]。脾脏是单核巨噬细胞的重要来源之一,同时也是肿瘤免疫耐受发生的部位,在肿瘤进展期切除脾脏可以减少肿瘤组织中单核巨噬细胞的数量,提高CD8+T细胞的细胞毒性,抑制肿瘤的生长[8-9]。由此可见,脾脏参与了肿瘤的进展,但脾脏在慢性应激促肿瘤进展中的作用尚不清楚。因此,本研究旨在探讨脾脏在慢性应激促肝癌进展中的作用。
1 材料与方法
1.1 实验动物 6~8周龄雄性C57BL/6小鼠28只,体重(20±2)g,为SPF级,购买于西安交通大学医学部实验动物中心,并饲养于西安交通大学医学部实验动物中心SPF标准动物房内。动物实验经西安交通大学医学部伦理委员会批准通过。
1.2 细胞株及细胞培养 H22细胞株购自中国典型培养物保藏中心。H22细胞培养于含有10%胎牛血清(购自Gibco)、1%青霉素-链霉素溶液(购自碧云天公司)的1640完全培养基中(购自Hyclone公司),在5%CO2、37 ℃饱和湿度的细胞培养箱中培养24 h后,用15 mL离心管收集起来,于1 000 r/min 常温离心5 min,弃掉上清,用生理盐水重悬,备用。
1.3 主要抗体 大鼠抗小鼠PE/Cyanine7-labeled CCR2、大鼠抗小鼠PE-labeled Ly6C、大鼠抗小鼠CD16/32、大鼠抗小鼠APC-labeled CD11b、大鼠抗小鼠Brilliant Violet 421TM-labeled CD45抗体购自美国Biolegend公司。
1.4 慢性束缚应激荷瘤小鼠模型的建立 待小鼠适应环境2周后,慢性应激小鼠给予束缚应激,2 h/d,应激方法是将小鼠水平束缚在通气的50 mL离心管中。应激5 d后皮下接种H22肝癌细胞建立皮下瘤模型。其具体方法为:将上述复苏并培养了24 h后的H22肝癌细胞用生理盐水重悬,并调整H22细胞浓度为1×107个/mL,吸取0.1 mL细胞悬液,无菌注射到小鼠腹腔内,等待小鼠出现大量腹腔积液后,无菌取出小鼠腹腔积液至15 mL离心管中,用生理盐水洗涤2次,每次洗后1 000 r/min离心5 min,去上清,用12 mL生理盐水重悬细胞,并将H22肝癌细胞稀释至5×106个/mL。用1 mL注射器小鼠左侧腹股沟处30度角度缓慢刺入皮下,并斜行进针1 cm左右后注入100 μL H22细胞悬液,见注射部位出现小皮丘,无漏液,证明注射成功。缓慢拔出注射器,用棉签轻压进针口1 min左右。接种肿瘤后继续应激21 d。
1.5 脾切除及假手术 为了排除手术的影响,于慢性应激前2周行脾切除或假手术,在紫外线灭菌后的环境下,腹腔注射4%水合氯醛(0.01 mL/g体重)麻醉小鼠,备皮并用碘伏消毒3遍,取腹部正中切口长约0.8 cm,充分暴露脾脏,分别用缝合线结扎脾脏上极和下极的血管后剪掉血管,最后将脾脏游离下来并摘除。检测血管结扎处是否有出血后,逐层缝合腹腔,碘伏消毒皮层后将小鼠侧卧保温复苏。假手术仅打开腹腔,不做脾切除术,直接缝合腹腔。
1.6 外周血及肿瘤单细胞悬液制备 小鼠接种肿瘤3周后用眼球取血法采血约1.0 mL于乙二胺四乙酸抗凝管中备用,并取其肿瘤组织称重。将上述所取乙二胺四乙酸抗凝血加入5 mL红细胞裂解液充分混匀,冰上孵育5 min,用含2 %胎牛血清的PBS 10 mL终止,4 ℃下350 g离心5 min,取沉淀细胞并用1 mL PBS重悬,计数,调细胞浓度为1×107~2×107个/mL,放置于冰上,备用。将获取的肿瘤组织用眼科剪剪碎,并加入DNaseⅠ(购自上海生工生物)和Ⅳ型胶原酶(购自上海生工生物),使其终浓度分别为1.5 g/L和3 g/L,于37 ℃下孵育30 min,并用200目尼龙网过滤,以获得单个细胞,4 ℃下350 g离心5 min,弃去上清液取沉淀细胞;用1 mL PBS重悬,计数,调细胞浓度为1×107~2×107个/mL,放置于冰上,备用。
1.7 流式细胞术检测肿瘤和外周血CD11b+Ly6C+CCR2+单核细胞的比例 吸取100 μL上述制备的细胞悬液加入流式管中,用抗大鼠抗小鼠CD16/32抗体4 ℃孵育15 min,按说明书加入适量的CCR2、Ly6C、CD11b、CD45抗体,4 ℃避光孵育30 min,后加入含1%胎牛血清的PBS 2 mL,4 ℃下350 g离心5 min,弃去上清液;重复1次,最后加入0.5 mL PBS重悬,用FACS Canto Ⅱ flow cytometer(美国BD公司)检测结果。
1.8 统计学方法 应用SPSS 13.0统计学软件分析数据。计量资料比较采用t检验、单因素方差分析和SNK-q检验。P<0.05为差异有统计学意义。
2 结 果
2.1 慢性应激对肝癌进展的影响 采取接种肿瘤前连续束缚应激5 d,接种肿瘤后再连续束缚应激21 d的慢性应激模型探讨慢性应激对肝癌进展的影响(图1),结果显示,对照组肿瘤重量为(0.109±0.040) g,应激组肿瘤重量为(0.283±0.047) g,应激组肿瘤重量明显大于对照组,差异有统计学意义(t=2.810,P=0.023)。
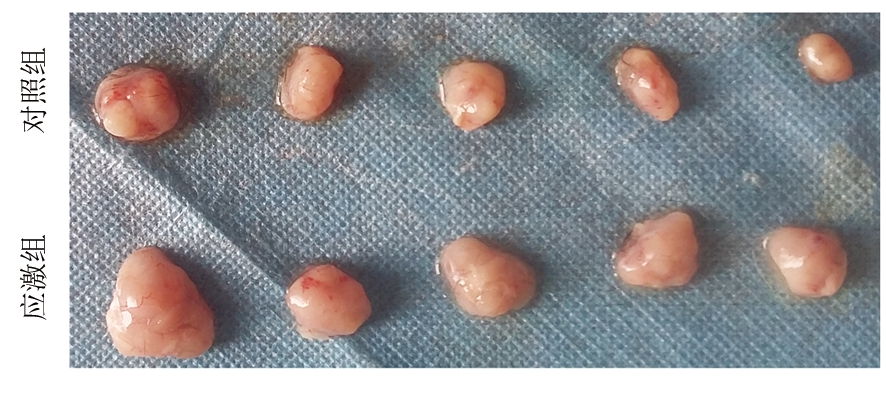
图1 慢性应激对肝癌进展的影响
Figure 1 The impact of chronic stress on hepatic carcinoma progression
2.2 慢性应激对CD11b+Ly6C+CCR2+单核细胞的影响 因CD11b+Ly6C+CCR2+单核细胞能促进肿瘤的进展,进一步用流式细胞术检测慢性应激对荷瘤小鼠外周血和肿瘤组织CD11b+Ly6C+CCR2+单核细胞比例的影响。结果显示,应激组外周血CD11b+Ly6C+CCR2+单核细胞的比例明显高于对照组,差异有统计学意义(P<0.05,图2);对照组和应激组肿瘤组织CD11b+Ly6C+CCR2+单核细胞的比例差异无统计学意义(P>0.05)。见表1。
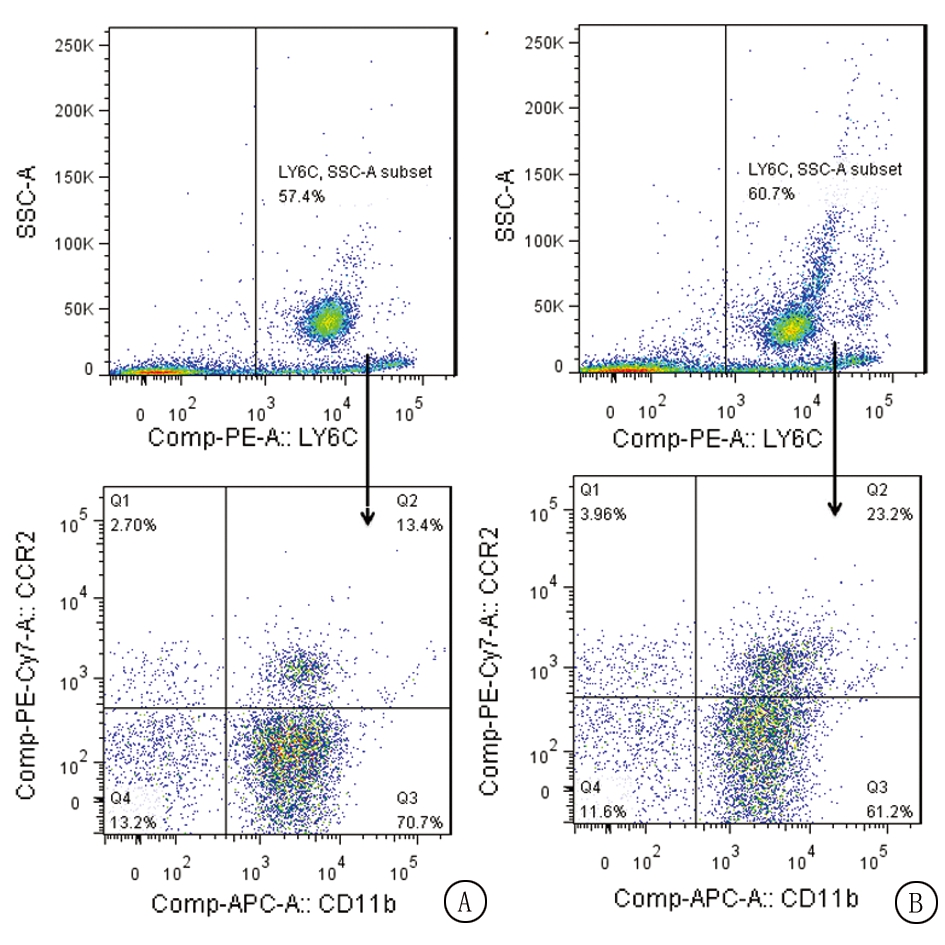
图2 慢性应激对外周血CD11b+Ly6C+CCR2+单核细胞的影响
A.对照组外周血CD11b+Ly6C+CCR2+单核细胞代表性流式图;B.应激组外周血CD11b+Ly6C+CCR2+单核细胞代表性流式图
Figure 2 The impact of chronic stress on the proportion of CD11b+Ly6C+CCR2+ monocyte in peripheral blood
表1 慢性应激对荷瘤小鼠外周血和肿瘤组织
CD11b+Ly6C+CCR2+单核细胞的影响
Table 1 The impact of chronic stress on the proportion of
CD11b+Ly6C+CCR2+ monocyte in the peripheral blood
and tumor tissue of tumor-bearing mice![]()
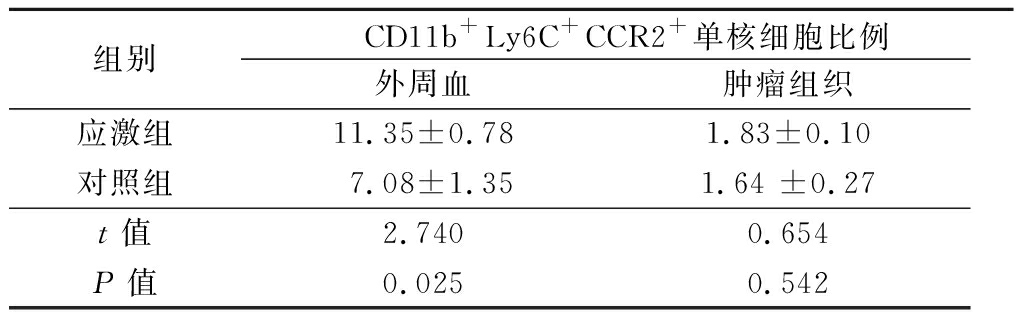
组别CD11b+Ly6C+CCR2+单核细胞比例外周血肿瘤组织应激组11.35±0.781.83±0.10对照组7.08±1.351.64 ±0.27t值2.7400.654P值0.0250.542
2.3 脾脏在慢性应激促肝癌进展中的作用 用脾切除的手段探讨脾脏在慢性应激促肝癌进展中的作用。结果显示,假手术应激组肿瘤重量明显高于假手术组和脾切除应激组,差异有统计学意义(P<0.05), 假手术组和脾切除应激组肿瘤重量差异无统计学意义(P>0.05)。见表2。
表2 脾脏在慢性应激促肝癌进展中的作用
Table 2 The role of the spleen in progression of hepatic
carcinoma induced by chronic stress![]()
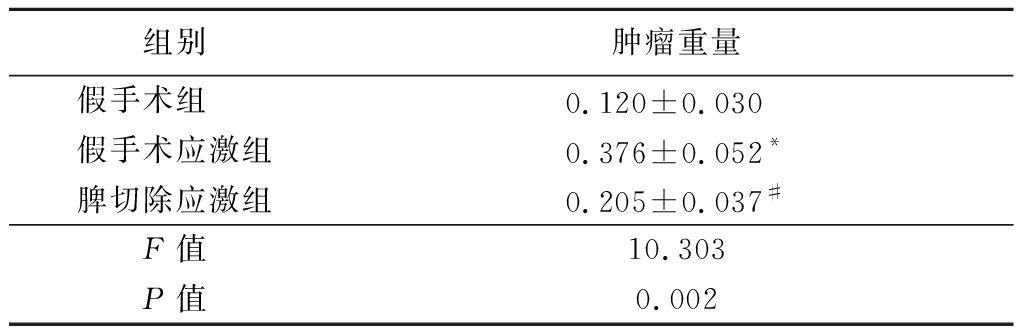
组别肿瘤重量假手术组 0.120±0.030 假手术应激组0.376±0.052*脾切除应激组0.205±0.037#F值10.303P值0.002
*P值<0.05与假手术组比较 #P值<0.05与假手术应激组比较(SNK-q检验)
2.4 脾切除对慢性应激荷瘤小鼠CD11b+Ly6C+CCR2+单核细胞的影响 检测脾切除对慢性应激荷瘤小鼠外周血和肿瘤组织CD11b+Ly6C+CCR2+单核细胞比例的影响。结果显示,与假手术组(7.65±0.78)%相比,假手术应激组外周血CD11b+Ly6C+CCR2+单核细胞的比例明显高于假手术组和脾切除应激组,差异有统计学意义(P<0.05),假手术组和脾切除应激组外周血CD11b+Ly6C+CCR2+单核细胞的比例差异无统计学意义(P>0.05)。假手术组、假手术应激组和脾切除应激组肿瘤组织CD11b+Ly6C+CCR2+单核细胞的比例差异无统计学意义(P>0.05)。见表3。
表3 脾切除对慢性应激荷瘤小鼠
CD11b+Ly6C+CCR2+单核细胞的影响
Table 3 The impact of splenectomy on the proportion of
CD11b+Ly6C+CCR2+ monocyte in chronic
stressed tumor-bearing mice![]()
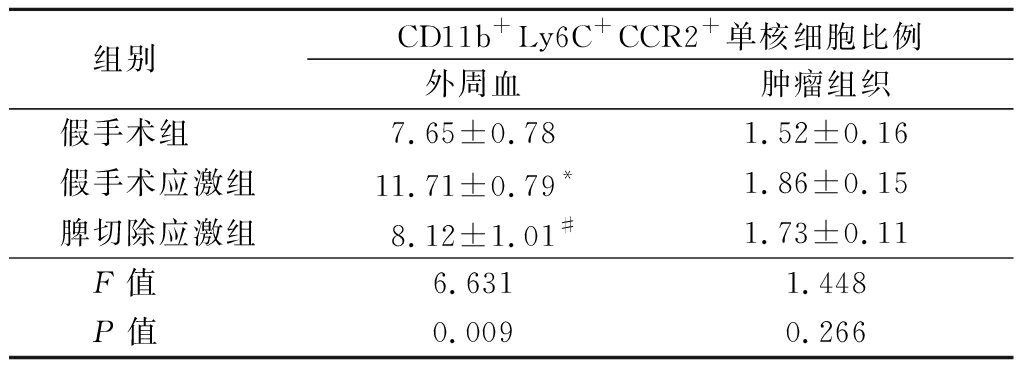
组别 CD11b+Ly6C+CCR2+单核细胞比例外周血肿瘤组织假手术组 7.65±0.781.52±0.16假手术应激组11.71±0.79*1.86±0.15脾切除应激组8.12±1.01#1.73±0.11F值 6.6311.448P值 0.0090.266
*P值<0.05与假手术组比较 #P值<0.05与假手术应激组比较(SNK-q检验)
3 讨 论
研究证实,慢性应激能通过抑制肿瘤的免疫微环境促进肿瘤进展[10]。高表达CCR2的单核细胞是肿瘤免疫微环境的重要组成部分,其分化为TAM,而TAM通过多种途径促进肿瘤进展[4]。慢性应激通过增加体内CCR2+单核细胞的比例促进肿瘤进展。Armaiz-Pena等[3]研究表明,慢性束缚应激通过交感神经系统上调肿瘤细胞表达CCL2,进而趋化单核巨噬细胞到肿瘤组织促进肿瘤生长;Chen等[5]研究结果表明,慢性应激通过交感神经系统上调肺组织表达CCL2,从而通过CCL2-CCR2通路趋化单核巨噬细胞到转移灶,进而促进乳腺癌的肺转移。本研究证实,慢性束缚应激促进肝癌进展并增加外周血CD11b+Ly6C+CCR2+单核细胞的比例,由此可见,慢性应激可能通过增加CD11b+Ly6C+CCR2+单核细胞的比例促进肝癌进展,但其具体机制需要进一步研究。
脾脏是机体最大的外周免疫器官,也是重要的髓外造血部位,参与多种疾病的发生发展,比如肿瘤、肝炎肝硬化、心脑血管疾病等[11-14]。脾脏对肿瘤的作用还未有定论,有的研究表明切除脾脏能促进肿瘤进展[15-16],有的研究表明切除脾脏对肿瘤的生长没有影响[17-18]。前期研究结果显示,脾切除能够抑制肿瘤进展[19-20]。不同的实验结果可能是由不同的动物模型和不同的切脾时间造成的,其具体机制还有待进一步研究。最近研究结果显示,脾脏作为一个重要的髓外造血的器官,是单核巨噬细胞的重要来源之一,荷瘤小鼠脾脏显著增大,脾脏同时是肿瘤免疫耐受的发生部位[8,21]。切除脾脏能抑制肿瘤的生长和减少单核巨噬细胞的数量[8-9]。前期研究表明,肝纤维化模型大鼠较正常大鼠脾脏中CCR2+单核巨噬细胞数量显著增加,肝纤维化模型大鼠切脾后肝脏单核巨噬细胞数量降低,说明脾脏是CCR2+单核细胞的重要来源之一[22]。慢性应激能促进脾脏中的单核巨噬细胞分布到外周器官,而慢性应激能招募脾脏中的单核细胞到小鼠大脑[23-25]。本研究结果显示,在造模前14 d切除脾脏能减缓慢性应激诱导的肿瘤生长,也能显著减少慢性应激小鼠外周血CD11b+Ly6C+CCR2+单核细胞的比例。由此可见,脾脏在慢性应激促肿瘤进展过程中起促进作用,慢性应激增加外周血CD11b+Ly6C+CCR2+单核细胞可能来源于脾脏。
[1] McGregor BA,Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer:a review of stress pathways and biological mediators [J]. Brain Behav Immun,2009,23(2):159-166.
[2] Bucsek MJ,Qiao G,MacDonald CR,et al. beta-Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy [J]. Cancer Res,2017,77(20):5639-5651.
[3] Armaiz-Pena GN,Gonzalez-Villasana V,Nagaraja AS,et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth [J]. Oncotarget,2015,6(6):4266-4273.
[4] Qian B,Li J,Zhang H,et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis [J]. Nature,2011, 475(7355):222-225.
[5] Chen H,Liu D,Guo L,et al. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling [J]. J Pathol,2018,244(1):49-60.
[6] Kristinsson SY,Gridley G,Hoover RN,et al. Long-term risks after splenectomy among 8,149 cancer-free American veterans:a cohort study with up to 27 years follow-up[J]. Haematologica,2014,99(2):392-398.
[7] Lv X,Yang F,Guo X,et al. Hypersplenism is correlated with increased risk of hepatocellular carcinoma in patients with post-hepatitis cirrhosis[J]. Tumour Biol,2016,37(7):8889-8900.
[8] Cortez-Retamozo V,Etzrodt M,Newton A,et al. Origins of tumor-associated macrophages and neutrophils[J]. Proc Natl Acad Sci U S A,2012,109(7):2491-2496.
[9] Ugel S,Peranzoni E,Desantis G,et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen[J]. Cell Rep,2012,2(3):628-639.
[10] Jiang W,Li Y,Li ZZ,et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling [J]. Brain Behav Immun,2019,80:825-838.
[11] Dragomir M,Petrescu DGE,Manga GE,et al. Patients after splenectomy:old risks and new perspectives[J]. Chirurgia(Bucur),2016,111(5):393-399.
[12] 李宗芳,周蕊,任松,等. 脾脏与肝病的研究进展[J]. 国际外科学杂志,2012,39(4):217-221.
[13] 张澍,李宗芳. 脾脏功能与脾脏外科研究现状与展望[J]. 中华实验外科杂志,2014,31(2):231-233.
[14] Li L,Duan M,Chen W,et al. The spleen in liver cirrhosis:revisiting an old enemy with novel targets [J]. J Transl Med,2017,15(1):111-121.
[15] Prehn RT. The paradoxical effects of splenectomy on tumor growth[J]. Theor Biol Med Model,2006,3:23.
[16] Higashijima J,Shimada M,Chikakiyo M,et al. Effect of splenectomy on antitumor immune system in mice[J]. Anticancer Res,2009,29(1):385-393.
[17] Soda K,Kawakami M,Takagi S,et al. Splenectomy before tumor inoculation prolongs the survival time of cachectic mice[J]. Cancer Immunol Immunother,1995,41(4):203-209.
[18] Imai S,Nio Y,Shiraishi T,et al. Effects of splenectomy on pulmonary metastasis and growth of SC42 carcinoma transplanted into mouse liver[J]. J Surg Oncol,1991,47(3):178-187.
[19] 李宝华,黄娜,陈海燕,等.肝癌小鼠脾脏免疫细胞的变化及其意义[J].现代肿瘤医学,2018,26(13):1980-1985.
[20] Li B,Zhang S,Huang N,et al. CCL9/CCR1 induces myeloid derived suppressor cell recruitment to the spleen in a murine H22 orthotopic hepatoma model[J]. Oncol Rep,2019,41(1):608-618.
[21] 程馥艳,刘锡文,徐晓武.三七总皂苷对人肝癌细胞HepG2移植瘤增殖的影响及其机制的研究[J].河北医科大学学报,2011,32(4):447-449.
[22] Li L,Wei W,Li Z,et al. The Spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis[J]. Cell Physiol Biochem,2018,51(2):557-574.
[23] Wohleb ES,McKim DB,Shea DT,et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain[J]. Biological Psychiatry,2014,75(12):970-981.
[24] McKim DB,Patterson JM,Wohleb ES,et al. Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat[J]. Biological Psychiatry,2016,79(10):803-813.
[25] Jiang W,Li Y,Sun J,et al. Spleen contributes to restraint stress induced changes in bloodleukocytes distribution[J]. Sci Rep,2017,7(1):6501.