股腘动脉硬化闭塞症作为血管外科常见病之一,间歇性跛行、静息痛、甚至发生溃疡或坏死等临床表现,严重影响患者生活治疗及身心健康,甚或危及生命。随着腔内技术和耗材不断发展,腔内治疗股腘动脉硬化病变已成为临床医师首选治疗方案[1-4],但传统经皮腔内血管成形术(percutaneous transluminal angioplasty,PTA)术后短期内再狭窄严重影响其治疗效果,而对于股腘动脉特定的血流动力学特点,尤其受到强大肌肉力量影像的特性,支架作为永久性植入物,很难保证其良好的远期通畅率,而且一旦出现支架内闭塞可能造成更加严重而棘手的下肢缺血加重,甚至走向肢体缺血坏死,而限制其广泛应用[5-7],因此目前临床主要将其作为“补救性支架”应用。药物涂层球囊(drug coated balloon,DCB)避免了植入异物,且可抑制内膜过度增生,其临床疗效也得到证实[8-12]。但DCB无法解决斑块弹性回缩,而且会增加限流性夹层发生风险[13],且对于过度钙化病变,会影响药物到达内膜的浓度及计量,从而影响疗效[14]。本研究尝试应用双导丝球囊进行预扩张,充分准备血管床,随后应用药物洗脱球囊治疗股腘动脉硬化闭塞症,在降低并发症发生率的同时,降低管腔再干预率。
1 资料与方法
1.1 一般资料 选择2018年6月—12月我院收治的股腘动脉缺血性疾病患者40 例的临床资料,对照组20例,观察组20例,年龄48~86岁,平均(65.8±8.1)岁。40例患者术前行下肢动脉超声及CTA 明确诊断,均顺行真腔开通病变,完成手术治疗。2组一般资料和临床特点比较差异无统计学意义(P<0.05),具有可比性。见表1,2。
表1 2组一般资料比较
Table 1 Comparision of general information between two groups (n=20)
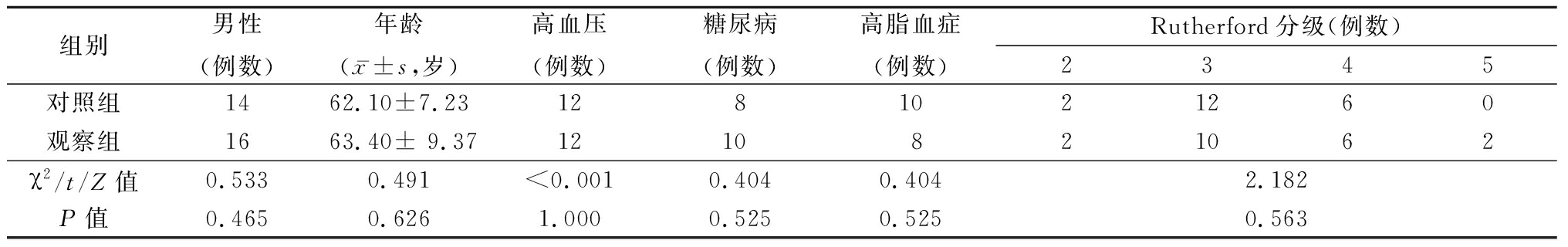
组别男性(例数)年龄(x-±s,岁)高血压 (例数) 糖尿病 (例数) 高脂血症(例数) Rutherford分级(例数) 2345对照组1462.10±7.231281021260观察组1663.40± 9.371210821062χ2/t/Z值0.5330.491<0.0010.4040.4042.182P值 0.4650.6261.0000.5250.5250.563
表2 2组病变长度及狭窄程度
Table 2 The length of lesions and the degree of
stenosis in two groups![]()
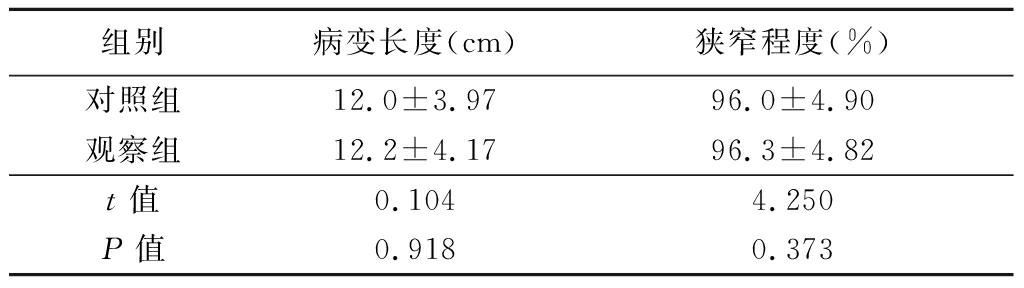
组别病变长度(cm)狭窄程度(%)对照组12.0±3.9796.0±4.90观察组12.2±4.1796.3±4.82t值0.1044.250P值0.9180.373
1.2 治疗方法
1.2.1 术前、术后护理 所有患者在控制原发病的基础上,术前给予抗血小板、抗凝、调脂、扩血管等药物辅助治疗。术后继续给予抗血小板、抗凝、调脂、扩血管等综合治疗1周。出院后给予抗血小板、调脂药物治疗。
1.2.2 腔内介入治疗应用 2%利多卡因进行局部浸润麻醉,应用Seldinger 技术行股动脉穿刺,根据术前影响结果选择最佳入路,本组病例均为长段股腘动脉病变,均行对侧股动脉逆行穿刺,穿刺成功后置入6F导管鞘,借助数字减影血管造影(digital subtraction angiography,DSA)明确病变情况,导管配合导丝真腔开通病变段,开通技巧可参考本中心已发表文献[15],开通后造影明确真腔,膝下动脉造影至少有一条通畅的流出道,将导丝选入远端血管。全身肝素化后,对照组应用PBA进行预扩张,对照组应用双导丝球囊进行预扩张。双导丝球囊进行加压需每半分钟增加1 atm(1 atm=101.325 kPa),直至球囊成形良好,持续扩张3 min,缓慢释放球囊。预扩张完成后造影观察残余狭窄及有无限流性夹层、远端栓塞出现,残余狭窄要求<30%,而出现限流性夹层需置入“补救性支架”,出现远端栓塞需给予吸栓、溶栓等治疗。
1.3 评价指标 记录患者术中相关并发症发生情况,如远端栓塞、限流性夹层等;分别监测患肢术前、术后3、6、12个月时踝肱指数(ankle brachial index,ABI)。对患肢术前、术后1年进行Rutherford分级[16];记录截肢率、死亡率。
1.4 统计学方法 应用SPSS 18.0 统计软件分析数据。计量资料采用独立样本t检验和采用重复测量方差分析,计数资料采用 χ2 检验和秩和检验。P<0.05为差异有统计学意义。
2 结 果
术中发生远端栓塞2例,观察组1例,对照组1例。观察组术中应用溶栓药及扩张血管药后复流,考虑紫杉醇粉末栓塞。而对照组出现斑块栓塞,给予再扩张,最终因远端流出道消失,出现急性缺血,给予溶栓后出现前足坏疽,最终截肢。对照组扩张完成后出现限流性夹层1例,给予植入“补救性支架”1枚。
40例患者均获得随访,2组病例ABI均较术前升高,术后3、6、12个月呈逐渐降低的趋势,2组在组间、时点间、组间·时点间交互作用差异均有统计学意义(P<0.05)。见表3。
表3 2组ABI比较
Table 3 Comparion of ABI between two groups![]()
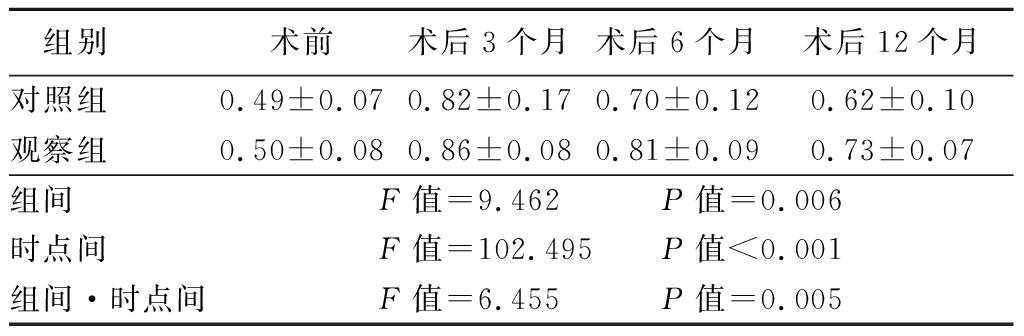
组别 术前术后3个月术后6个月术后12个月对照组 0.49±0.070.82±0.170.70±0.120.62±0.10观察组 0.50±0.080.86±0.080.81±0.090.73±0.07组间 F值=9.462 P值=0.006时点间 F值=102.495 P值<0.001组间·时点间F值=6.455 P值=0.005
3 讨 论
外周动脉疾病(peripheral artery disease,PAD)作为目前临床讨论热点问题之一,全世界有超过2亿人患有PAD,其中70岁以上患者约占15%[17]。随着腔内技术及器械的不断发展,介入治疗已经成为首选治疗方案,但是术后再狭窄问题一直是困扰临床医师的一大难题,尤其是股腘动脉硬化闭塞症[13]。分析其机制,主要包括三点:斑块弹性回缩、血栓形成、内膜过度增生。临床通过植入裸支架来预防斑块弹性回缩及内膜过度增生,通过随机对照试验证实,其再干预率较传统PTA有所改善[18-19]。但其作为永久性植入物,可致支架内血栓形成及血流动力学发生改变,从而导致支架再闭塞甚至断裂,从而限制其广泛应用[5-7],因此目前临床主要将其作为“补救性支架”应用。
DCB无需植入异物,通过输送紫杉醇到达病变血管,紫杉醇通过其亲脂性可更好的转移到动脉壁,防止血流冲刷,进而通过稳定微管,阻止有丝分裂,抑制细胞DNA的合成,有效抑制平滑肌增殖、迁移和细胞外基质形成这三个机制来抑制内膜过度增生,从而降低术后再狭窄率。与PBA相比,其安全有效性也得到临床认可[8-12]。但DCB无法解决斑块弹性回缩,而且会增加限流性夹层发生风险,且对于过度钙化病变,紫杉醇无法更好地渗透于病变血管内膜表面,从而影响其疗效。为对斑块进行预处理,临床提出应用腔内减容术结合药物洗脱球囊治疗股腘动脉硬化闭塞证,并取得较好临床疗效[20-22],但基于腔内减容耗材的过于昂贵,限制了它的广泛应用,且其疗效是与减容有关,抑制内膜增生有关,还是与二者共同作用有关,尚缺乏对照研究。本中心试图应用双导丝球囊结合药物洗脱球囊治疗股腘动脉过度钙化病变。
双导丝球囊相对于PBA而言,具有如下优点:低压球囊;可控性好;对血管内膜损伤小;对斑块起到纵向切割作用等,从而减少内膜炎性反应及内膜过度增生。此球囊在国外已得到广泛应用,并已得到证实其即刻效应与远期再干预率均优于PBA[23]。虽然双导丝球囊无法将斑块祛除体外,但其可有效改变斑块结构,将其重新塑性,从而增加紫杉醇到达病变内膜的药物浓度,从而增加其疗效。关于应用双导丝球囊结合DCB治疗股腘动脉硬化闭塞症已有报道[24],其安全性已得到证实,但其缺乏对照性研究。对此,本研究通过与PBA进行对比,通过分析术中并发症发生情况及术后再干预率及症状改善情况,来进一步验证双导丝球囊结合DCB治疗股腘动脉硬化闭塞症的安全有效性。
本研究中20例接受双导丝球囊预处理的患者,均未发生因斑块破裂导致远端栓塞及限流性夹层形成并发症,证实其相比应用PBA进行预处理更加安全。根据6、12个月随访结果,观察组ABI及中期再干预率均优于对照组,且患者症状均得到明显改善。
但该研究也存在不足之处,如病例数较少,统计结果缺乏充分的代表性,且缺乏远期随访结果,对于远期应用效果有待进一步随访。因此,我们将进一步验证双导丝球囊结合DCB治疗下肢动脉硬化闭塞症的安全有效性,并借助动物实验,来观察双导丝球囊对斑块形态及结构的影响,以及紫杉醇到达病变血管内膜的药物浓度,根据不同类型斑块来调整载药球囊药物浓度,从而达到最佳治疗效果。
综上所述,对于股腘动脉硬化闭塞症,单纯球囊扩张效果不佳,即刻效果及远期再通率均较差,虽然腔内减容结合药物洗脱球囊为临床带来新的治疗思路,但基于其治疗费用昂贵,限制其广泛推广,且目前尚缺乏对照研究。对比,本中心尝试应用双导丝球囊结合DCB治疗股腘动脉硬化闭塞症,虽无法将斑块祛除体外,但凭借其优点可较好地塑形血管,改变斑块结构,减轻斑块弹性回缩的同时,促使紫杉醇更多地渗透到病变血管内膜,从而达到抑制内膜过度增生疗效,降低再狭窄率。为临床治疗下肢动脉硬化闭塞症提供一种新的选择方案。
[1] Giannopoulos S,Armstrong EJ. Newly approved devices for endovascular treatment of femoropopliteal disease:a review of clinical evidence[J]. Expert Rev Cardiovasc Ther,2019,17(10):729-740.
[2] Kokkinidis DG,Armstrong EJ. Current developments in endovascular therapy of peripheral vascular disease[J]. J Thorac Dis,2020,12(4):1681-1694.
[3] Anantha-Narayanan M,Love K,Nagpal S,et al. Safety and efficacy of paclitaxel drug-coated balloon in femoropopliteal in-stent restenosis[J]. Expert Rev Med Devices,2020,17(6):533-539.
[4] Chen PL,Lin TC,Chen IM. Hybrid viabahn-assisted bypass for long femoro-popliteal occlusive disease-midterm results[J]. Circ J,2018,82(8):2160-2164.
[5] Anantha-Narayanan M,Love K,Nagpal S,et al. Safety and efficacy of paclitaxel drug-coated balloon in femoropopliteal in-stent restenosis[J]. Expert Rev Med Devices,2020,17(6):533-539.
[6] Stone D. A literature review of the efficacy of debulking devices for in-stent restenosis of the femoropopliteal artery[J]. J Vasc Surg,2020,72(1):367.
[7] Guo S,Zhang Z,Wang L,et al. Six-month results of stenting of the femoropopliteal artery and predictive value of interleukin-6:Comparison with high-sensitivity C-reactive protein[J]. Vascular,2020,28(6):715-721.
[8] Schroê H,Holden AH,Goueffic Y,et al. Stellarex drug-coated balloon for treatment of femoropopliteal arterial disease-The ILLUMENATE Global Study:12-Month results from a prospective,multicenter,single-arm study[J]. Catheter Cardiovasc Interv,2018,91(3):497-504.
[9] Tepe G,Micari A,Keirse K,et al. Drug-coated balloon treatment for femoropopliteal artery disease:the chronic total occlusion cohort in the IN.PACT Global Study[J]. JACC Cardiovasc Interv,2019,12(5):484-493.
[10] 张军,林瑞敏,刘军,等.药物涂层球囊治疗下肢动脉硬化闭塞症的临床观察[J].河北医科大学学报,2019,40(2):178-180,197.
[11] Secemsky EA,Kundi H,Weinberg I,et al. Association of survival with femoropopliteal artery revascularization with drug-coated devices[J]. JAMA Cardiol,2019,4(4):332-340.
[12] Ouriel K,Adelman MA,Rosenfield K,et al. Safety of paclitaxel-coated balloon angioplasty for femoropopliteal peripheral artery disease[J]. JACC Cardiovasc Interv,2019,12(24):2515-2524.
[13] Magnus B,Shalva C,Michael K. Treatment of femoro-popliteal lesions with scoring and drug-coated balloon angioplasty:12-month results of the DCB-Trak registry[J]. Diagn Interv Radiol,2018,24(3):153-157.
[14] Cassese S,Byrne R A,Ott I,et al. Paclitaxel-coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease a meta-analysis of randomized trials[J]. Circ Cardiovasc Interv,2012,5(4):582-589.
[15] 袁涛,高翔,张楠,等.股腘动脉长段闭塞的介入治疗[J].血管与腔内血管外科杂志,2016,2(6):483-488.
[16] Conte MS,Bradbury AW,Kolh P,et al. Global vascular guidelines on the management of chronic limb-threatening ischemia[published correction appears in J Vasc Surg][J]. J Vasc Surg,2019,69(6S):3S-125S.e40.
[17] Kaplovitch E,Rannelli L,Anand SS. Antithrombotics in stable peripheral artery disease[J]. Vasc Med,2019,24(2):132-140.
[18] Koifman E,Lipinski MJ,Buchanan K,et al. Comparison of treatment strategies for femoro-popliteal disease:a network meta-analysis[J]. Catheter Cardiovasc Interv,2018,91(7):1320-1328.
[19] Iida O,Urasawa K,Komura Y,et al. Self-expanding nitinol stent vs percutaneous transluminal angioplasty in the treatment of femoropopliteal lesions:3-year data from the SM-01 Trial[J]. J Endovasc Ther,2019,26(2):158-167.
[20] Sauguet A,Philippart R,Honton B. Directional atherectomy with antirestenotic therapy for the treatment of no-stenting zones[J]. J Cardiovasc Surg(Torino),2019,60(2):198-204.
[21] Zhen Y,Chang Z,Wang C,et al. Directional atherectomy with antirestenotic therapy for femoropopliteal artery disease:a systematic review and meta-analysis[J]. J Vasc Interv Radiol,2019,30(10):1586-1592.
[22] Loffroy R,Chevallier O,Falvo N,et al. Combined hawk one directional atherectomy and paclitaxel-coated balloon angioplasty for isolated calcified popliteal artery lesion:a no-stent approach to lower extremity endovascular revascularization[J]. Quant Imaging Med Surg,2018,8(3):364-367.
[23] Poncyljusz W,Falkowski A,Safranow K,et al. Cutting-balloon angioplasty versus balloon angioplasty as treatment for short atherosclerotic lesions in the superficial femoral artery:randomized controlled trial[J]. Cardiovasc Interv Radiol,2013,36(6):1500-1507.
[24] Baumhäkel M,Chkhetia S,Kindermann M. Scoring- plus drug coated balloon in femoro-popliteal lesions - 6 months results of the DCB-Trak-Registry[J]. J Cardiol Ther,2016,4:8-12.