缺血性二尖瓣反流(ischemic mitral regurgitation,IMR)是由慢性冠状动脉疾病引起的常见并发症[1-2],且与心肌梗死(myocardial infarction,MI)的不良预后密切相关,使病死率翻倍,同时也会增加心力衰竭的风险[3-4]。经胸实时三维超声心动图通过定量分析二尖瓣的三维结构直观反映患者的二尖瓣变化,本研究通过经胸实时三维超声心动图对不同部位MI的IMR患者的二尖瓣构型进行研究,并分析IMR严重程度的影响因素,为临床治疗提供参考。
1 资料与方法
1.1 一般资料 选择2019年4月-2020年1月河北省保定市第一中心医院就诊的MI伴IMR患者64例。纳入标准:①经冠状动脉造影证实有明显的冠状动脉病变,常规超声检查有节段性室壁运动异常,以前壁MI及下壁MI为主;②伴有中度及以上的二尖瓣反流者,通过PISA法测量瓣口有效反流面积(effective regurgitant orifice area,EROA),0.2 cm2≤EROA<0.4 cm2为中度,EROA≥0.4 cm2为重度[5-6]。排除标准:①多部位MI;②器质性二尖瓣病变;③合并其他瓣膜病变;④先天性心脏病;⑤图像质量不佳者。根据梗死部位分为前壁MI组40例和下壁MI组24例。另选择健康志愿者45例纳入对照组,排除心脏相关疾病,并行超声心动图检查未见明显二尖瓣反流。3组性别、年龄、体重、体重指数、心率差异无统计学意义(P>0.05),具有可比性。见表1。
表1 3组一般资料比较
Table 1 Comparison of basic information among three groups![]()
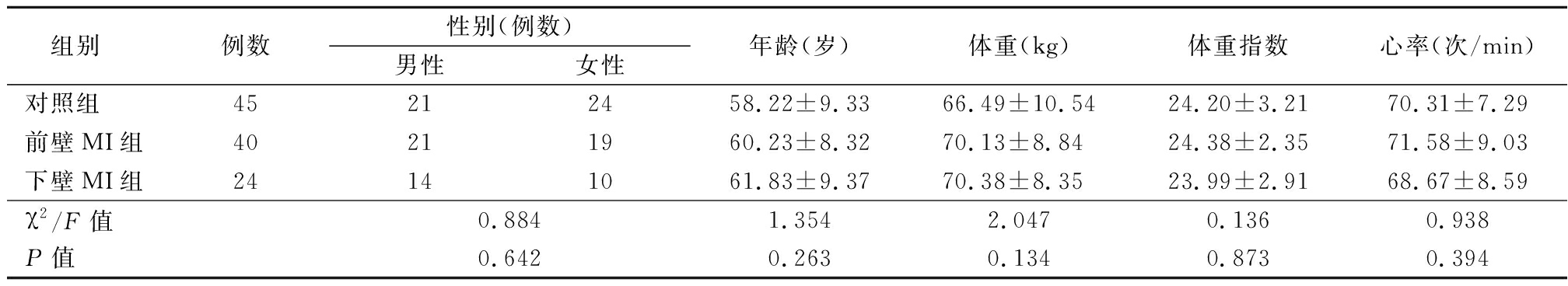
组别例数性别(例数)男性女性年龄(岁)体重(kg)体重指数 心率(次/min)对照组45212458.22±9.3366.49±10.5424.20±3.2170.31±7.29前壁MI组40211960.23±8.3270.13±8.8424.38±2.3571.58±9.03下壁MI组24141061.83±9.3770.38±8.3523.99±2.9168.67±8.59χ2/F值0.8841.3542.0470.1360.938P值0.6420.2630.1340.8730.394
本研究经医院医学伦理委员会批准通过,所有受检对象均知情同意并签署知情同意书。
1.2 仪器与方法 采用GE Vivid E9彩色多普勒超声诊断仪,4 V探头,频率1.5~4.0 MHz;M5S探头,频率1.5~4.6 MHz,EchoPAC工作站对存储的动态图像进行分析,Tom Tec软件处理二尖瓣参数。
1.2.1 左心室整体重塑参数 在心尖四腔、二腔二维图像中通过双平面辛普森法测量以下参数:左心室舒张末期容积(left ventricular end-diastolic volume,LVEDV)、左心室收缩末期容积( left ventricular end-systolic volume,LVESV)、左心室射血分数(left ventricularejection fraction,LVEF)。
1.2.2 三维二尖瓣几何参数 通过TomTec 软件分析获得二尖瓣环的参数:瓣环前后径(anterior-to-posterior diameter,DAP)、瓣环高度(annular height,AH)、联合处直径(commissural diameter,CD)、瓣环三维面积(three-dimensional annular area,A3D)、非平面角度(nonplanar angle,NPA)、幕状区容积(tenting volume,VTent)、幕状区高度(tenting height,HTent)、瓣环周长(three-dimensional annular circumference,AC)、后叶角度(posterior leaflet angle,θPost)。
1.3 重复性检验 由2位有经验的医师同时对随机抽出的10例患者用同样的方法测量DAP、NPA、HTent、A3D、CD、AC、AH、θPost、VTent,再由其中的1位医师在不同时间重复测量以上参数,计算组内相关系数(interclass correlation coeficient,ICC)。
1.4 统计学方法 应用SPSS 19.0统计软件分析数据。计量资料比较采用单因素方差分析和SNK-q检验;计数资料比较采用χ2检验,IMR严重程度的影响因素分析采用多元Logistic回归分析。P<0.05为差异有统计学意义。
2 结 果
2.1 3组左心室重塑参数比较 前壁MI组、下壁MI组LVESV、LVEDV明显大于对照组,LVEF明显小于对照组;前壁MI组LVESV、LVEDV明显大于下壁MI组,LVEF明显小于下壁MI组,差异有统计学意义(P<0.05)。见表2。
表2 3组左心室重塑参数比较
Table 2 Comparison of left ventricular remodeling
parameters among three groups![]()
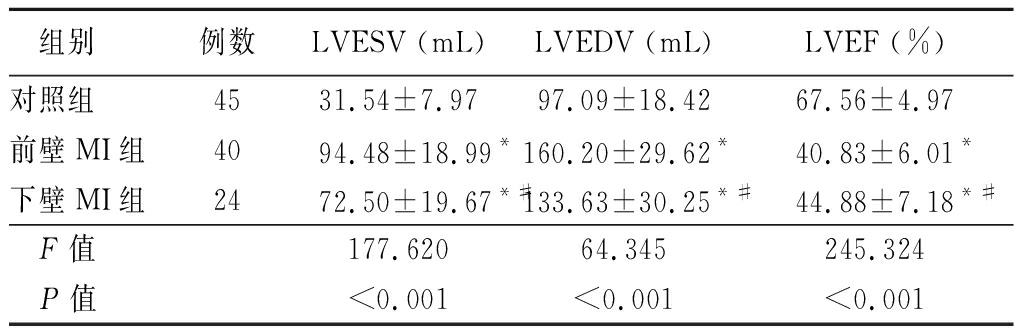
组别例数LVESV (mL) LVEDV (mL) LVEF (%) 对照组 4531.54±7.97 97.09±18.42 67.56±4.97前壁MI组4094.48±18.99*160.20±29.62*40.83±6.01*下壁MI组2472.50±19.67*#133.63±30.25*#44.88±7.18*# F值 177.620 64.345 245.324 P值<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与前壁MI组比较(SNK-q检验)
2.2 3组二尖瓣三维参数比较 前壁MI组和下壁MI组DAP、CD、A3D、NPA、HTent、VTent、AC、θpost大于对照组,AH小于对照组,差异有统计学意义(P<0.05)。前壁MI组CD、A3D、HTent、VTent大于下壁MI组,θpost小于下壁MI组,差异有统计学意义(P<0.05);前壁MI组和下壁MI组DAP、AH、NPA、AC差异无统计学意义(P>0.05)。见表3。
表3 3组二尖瓣三维参数比较
Table 3 Comparison of three dimensional parameters of mitral valve among three groups![]()
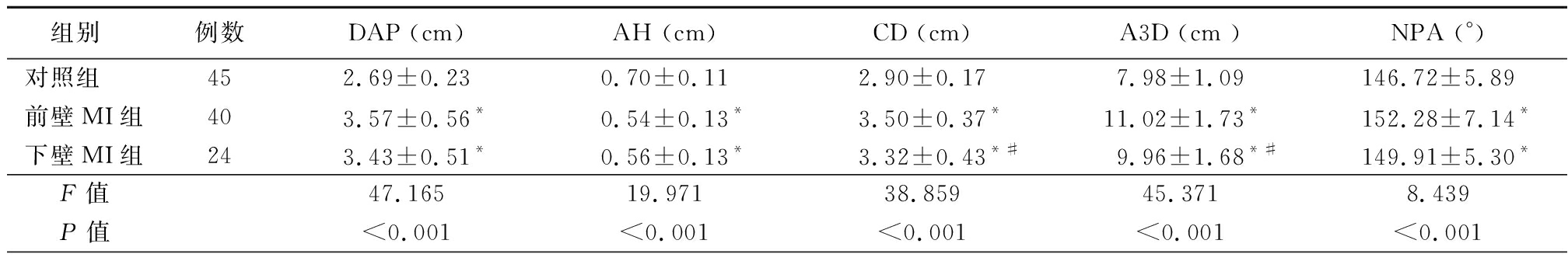
组别例数DAP (cm) AH (cm) CD (cm) A3D (cm )NPA (°)对照组452.69±0.230.70±0.112.90±0.17 7.98±1.09146.72±5.89前壁MI组403.57±0.56*0.54±0.13*3.50±0.37*11.02±1.73*152.28±7.14*下壁MI组243.43±0.51*0.56±0.13* 3.32±0.43*#9.96±1.68*#149.91±5.30* F值47.16519.971 38.85945.371 8.439 P值<0.001<0.001<0.001<0.001<0.001
表3 (续)
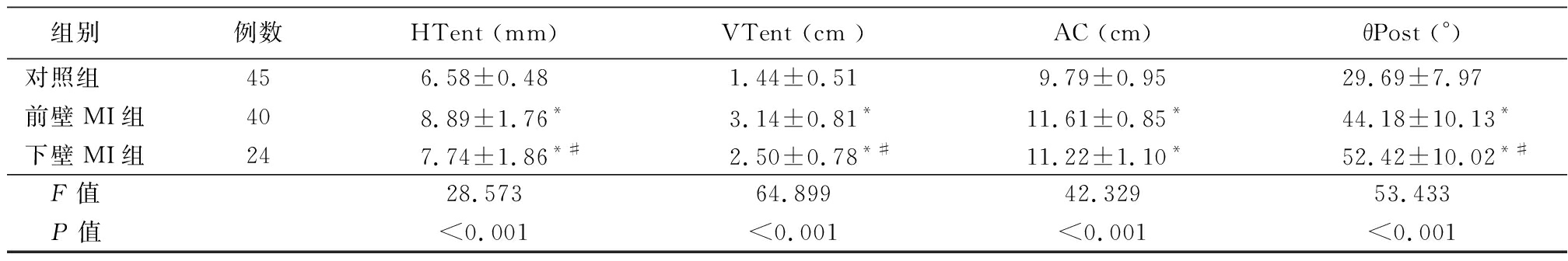
组别例数HTent (mm)VTent (cm )AC (cm)θPost (°)对照组456.58±0.481.44±0.519.79±0.9529.69±7.97前壁MI组408.89±1.76* 3.14±0.81*11.61±0.85* 44.18±10.13*下壁MI组247.74±1.86*#2.50±0.78*#11.22±1.10*52.42±10.02*# F值28.573 64.899 42.32953.433 P值<0.001<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与前壁MI组比较(SNK-q检验)
2.3 前壁MI组和下壁MI组不同反流程度发生率 下壁MI组重度IMR发生率大于前壁MI组,差异有统计学意义(P<0.05)。见表4。
表4 前壁MI组及下壁MI组不同程度反流的发生率
Table 4 The incidence of IMR in inferior myocardial
infarction and anterior myocardial infarction (例数,%)
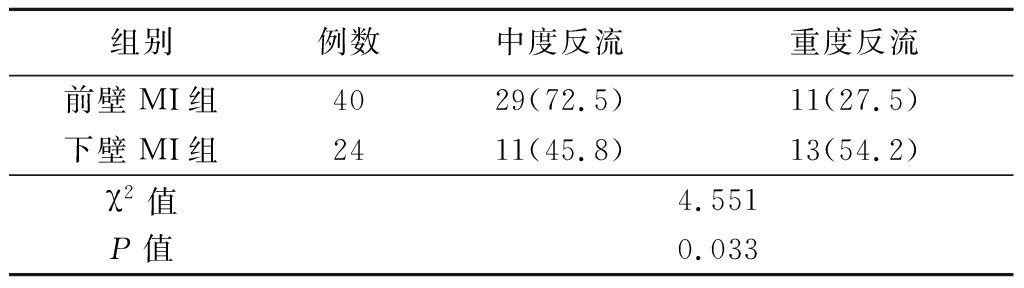
组别例数中度反流重度反流前壁MI组4029(72.5)11(27.5)下壁MI组2411(45.8)13(54.2)χ2值4.551P值0.033
2.4 病例组IMR严重程度的影响因素分析 以反流严重程度(中度=0,重度=1)为因变量,以LVESV(连续性变量)、LVEDV(连续性变量)、LVEF(连续性变量)、DAP(连续性变量)、AH(连续性变量)、CD(连续性变量) 、A3D(连续性变量)、NPA(连续性变量)、HTent(连续性变量)、VTent(连续性变量)、AC(连续性变量)、θpost(连续性变量)为自变量进行多元Logistic回归分析,结果显示,VTent、θpost是重度IMR的危险因素。见表5。
表5 重度IMR的影响因素
Table 5 Influencing factors of severe IMR

影响因素回归系数回归系数标准误 Wald χ2值 P值OR值95%CIVTent0.8690.4433.8590.0492.3851.002~5.678θpost0.0740.0364.1940.0411.0771.003~1.156
2.5 重复性检验 DAP、AH、CD、A3D、NPA、HTent、VTent、AC、θPost的观察者间ICC值为0.962、0.811、0.881、0.872、0.946、0.932、0.929、0.848、0.884,重复性较好。DAP、AH、CD、A3D、NPA、HTent、VTent、AC、θPost的观察者内ICC值为0.919、0.831、0.937、0.928、0.896、0.887、0.922、0.928、0.933,重复性较好。
3 讨 论
IMR与其他二尖瓣反流相比往往会产生较差的预后,病死率较高[7],充分了解IMR的形成机制对其治疗十分重要,前壁及下壁MI的IMR患者的二尖瓣构型改变及其形成机制的深入研究可以为临床治疗提供参考意义。左心室收缩作用在二尖瓣瓣叶表面的压力,乳头肌对二尖瓣的牵拉力以及瓣环对二尖瓣的作用力一起维持了瓣叶的正常闭合[8]。乳头肌、二尖瓣瓣环及左心室后壁对IMR的影响最大[9]。左心室重塑、乳头肌移位是IMR形成的主要原因[10]。
二维超声心动图不能全面的反映二尖瓣三维结构,经胸实时三维超声心动图与经食管三维超声心动图有较好的一致性,且操作简便,无禁忌证[11],可以为不同类型的患者提供术前二尖瓣的三维结构[12],从而为临床对患者制定合适的二尖瓣成形术策略提供参考。NPA反映瓣环“马鞍型”三维构型,马鞍型结构对维持瓣环对瓣叶的应力十分重要[13],前壁MI组和下壁MI组NPA均大于对照组,IMR患者瓣环鞍型结构趋于扁平,DAP、CD、A3D、AC均大于对照组,IMR患者瓣环明显扩张以往研究得出类似结论[14-15]。前壁MI组LVESV、LVEDV明显大于下壁MI组,LVEF小于下壁MI组,反映了前壁MI组左心室重塑程度大于下壁MI组。前壁MI组A3D、CD也明显大于下壁MI组,反映了前壁MI组的瓣环扩张程度较下壁MI组改变更明显,提示前壁MI所引起的IMR是在广泛左心室重塑的基础上发生的[16]。后叶角度可以反映后叶的牵拉情况,下壁MI组较前壁MI组θpost明显增大,提示下壁MI致后乳头肌向后移位,使得二尖瓣后叶被腱索过度牵拉所致。
经多元Logistic回归分析获得VTent、θpost是重度二尖瓣反流的危险因素,幕状区是综合二尖瓣装置多个独立几何因素改变而形成的三维立体结构,三维幕状区容积作为评价二尖瓣牵拉程度的三维几何参数,与二尖瓣反流严重与否密切相关[17]。以往研究报道,下壁MI患者重度二尖瓣反流发生率高于前壁MI患者[18],与本研究结果一致。下壁MI组VTent小于前壁VTent,但是重度反流发生率高于前壁MI组,其原因可能与后叶角度为重度二尖瓣反流的危险因素有关。以往研究提出后叶角度与反流量有很强的相关性(r=0.900,P=0.037)[19],本研究结论与之相似。
由于IMR形成机制复杂,外科对于IMR的治疗存在较大争议[20]。限制性瓣环成形术是临床比较认可的术式,限制性瓣环成形术通过降低瓣环前后径,从而使被牵拉的瓣叶可以覆盖二尖瓣瓣口,但是二尖瓣瓣环前半部分固定在主动脉瓣-心室膜上,导致限制性成形术对于瓣环的缩小是不平衡的,从而会加剧后叶被牵拉程度。以往研究提出后叶角度≥45 °是限制性瓣环成形术后复发的独立预测因素,对于下壁MI所致的后乳头肌移位产生对于后叶过度牵拉所致的IMR,可以考虑二尖瓣置换术或者并行手术。瓣环的选择对于瓣环成形术也是十分重要的,IMR患者瓣环形状随个体差异而不同[1],不同部位MI的二尖瓣构型不同应该纳入到二尖瓣成形术考虑范围之内。
本研究存在一定的局限性,样本量较小,仍需较大样本量进一步研究,本研究只探讨了前壁MI与下壁MI二尖瓣构型的改变,并未讨论其他部位MI对二尖瓣构型的影响,仍需进一步研究。
总之,本研究得出前壁MI与下壁MI的二尖瓣反流的形成机制不同。幕状区容积、后叶角度是IMR恶化的危险因素,对临床治疗具有一定的参考意义。
[1] Varma PK,Krishna N,Jose RL,et al. Ischemic mitral regurgitation[J]. Ann Card Anaesth,2017,20(4):432-439.
[2] B ez-Ferrer N,Izquierdo-G
ez-Ferrer N,Izquierdo-G mez MM,Marí-López B,et al. Clinical manifestations,diagnosis,and treatment of ischemic mitral regurgitation: a review[J]. J Thoracic Dis,2018,10(12):6969-6986.
mez MM,Marí-López B,et al. Clinical manifestations,diagnosis,and treatment of ischemic mitral regurgitation: a review[J]. J Thoracic Dis,2018,10(12):6969-6986.
[3] Bursi F,Enriquez-Sarano M,Nkomo VT,et al. Heart failure and death after myocardial infarction in the community:the emerging role of mitral regurgitation[J]. Circulation,2005,111(3):295-301.
[4] 付威,张魁,赵洋,等.缺血性二尖瓣反流的研究进展[J].心肺血管病杂志,2019,38(9):989-991.
[5] 潘翠珍,潘文志,周达新.二尖瓣反流介入治疗的超声心动图评价中国专家共识[J].中华超声影像学杂志,2019,28(1):1-6.
[6] O′Gara PT,Grayburn PA,Badhwar V,et al. 2017 ACC expert consensus decision pathway on the management of mitral regurgitation a report of the American college of cardiology task forceon expert consensus decision pathways [J]. J Am Coll Cardiol,2017,70(19):2421-2449.
[7] Agricola E,Oppizzi M,Maisano F,et al.Echocardiographic classification ofchronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern[J]. Eur J Echocardiogr,2004,5(5):326-334.
[8] 王芳,王婷,王婷婷,等.经食管三维超声定量评价器质性与缺血性二尖瓣反流时二尖瓣叶及瓣环形态变化[J].宁夏医学杂志,2019,41(6):514-516.
[9] 孟湘,刘昕.斑点追踪成像技术结合三维超声评价缺血性二尖瓣反流患者的乳头肌功能[J].中国医学影像学杂志,2017,25(3):203-207.
[10] 刘昕,孟湘.二维斑点追踪成像技术评价心肌梗死患者乳头肌功能变化的临床研究[J].中华超声影像学杂志,2017,26(5):398-402.
[11] 薛继平,吕虹,康春松,等.经胸与经食管三维超声评估健康人二尖瓣构型的研究[J].中华超声影像学杂志,2016,25(12):1013-1020.
[12] 董娟,康春松,王欢,等.经胸实时三维超声心动图在功能性二尖瓣反流中的应用[J].中国医学影像技术,2017,33(3):330-334.
[13] Jassar AS,Vergnat M,Jackson BM,et al. Regional annular geometry in patients with mitral regurgitation: implications for annuloplasty ring selection[J]. Ann Thorac Surg,2014,97(1):64-70.
[14] Morbach C,Bellavia D,Störk S,et al. Systolic characteristics and dynamic changes of the mitral valve in different grades of ischemic mitral regurgitation-insights from 3D transesophageal echocardiography[J]. BMC Cardiovasc Disord,2018,18(1):93.
[15] 刘从兵,贺亚琼,何金朋,等.经食管实时三维超声心动图定量评价二尖瓣复合体构型变化对缺血性二尖瓣反流的影响[J].中国超声医学杂志,2019,35(2):137-141.
[16] Vergnat M,Jassar AS,Jackson BM,et al. Ischemic mitral regurgitation: a quantitative three-dimensional echocardiographic analysis[J]. Ann Thorac Surg,2010,91(1):157-164.
[17] 陈昕,韩玉娜,曾燕玲,等.实时三维超声心动图评价二尖瓣穹窿容积对功能性二尖瓣反流的影响[J].中国超声医学杂志,2012,28(5):429-431.
[18] Lešniak-Sobelga A,Wicher-Muniak E,Kostkiewicz M,et al. Relationship between mitral leaflets angles,left ventricular geometry and mitral deformation indices in patients with ischemic mitral regurgitation: imaging by echocardiography and cardiac magnetic resonance[J]. Int J Cardiovasc Imaging,2012,28(1):59-67.
[19] Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications[J]. J Am Soc Echocardiogr,2011,24(7):707-719.
[20] 朱昌盛,王水云,崔颢,等.缺血性二尖瓣反流的外科治疗[J].中华胸心血管外科杂志,2018,34(7):438-440.