蛛网膜下腔出血(subarachnoid hemorrhage,SAH)是最常见的脑血管意外之一,致死致残率较高[1]。既往研究表明,SAH患者1周内的全因病死率高达40%[2]。除疾病本身外,SAH的并发症也是导致其不良预后的重要因素。急性肾损伤(acute kidney injury,AKI)作为SAH患者常见的严重并发症之一,可显著增加SAH患者的病死率,因此,如何改善SAH合并AKI患者的肾脏功能是急需解决的医学难题之一[3]。氢气作为一种选择性抗氧化剂,具有易穿透细胞膜,可以作用于亚细胞结构,通过参与多种信号通路的调控,选择性清除机体内氧自由基的特点[4]。既往研究表明,氢气能选择性的清除的·OH和ONOO-等能导致细胞损伤的强氧化剂,从而产生抗炎,抗凋亡的作用[5]。已有研究证实,吸入低浓度氢气能减轻SAH大鼠神经元损伤,减轻局灶性脑缺血再灌注损伤[6]。但关于氢气对于SAH导致的远隔器官损伤的保护作用尚不明确,本研究旨在探讨氢气对SAH致大鼠AKI的肾脏功能的保护作用机制。
1 材料与方法
1.1 一般资料 53只SPF级Sprague Dawley雄性大鼠,8~9周龄,体重300~350 g,购自北京维通利华实验动物有限公司(SYXK(京)2012-0036)。所有大鼠饲养于动物房,室温维持在(25±1) ℃,湿度维持(55±5)%,光照/黑暗周期为12 h/12 h。所有大鼠手术前均分笼适应性饲养1周,所有大鼠在实验过程中均参考国家卫生研究院发布的《实验动物护理和使用指南》的建议接受人类护理。
1.2 主要设备与试剂 七氟醚购于上海恒瑞医药有限公司;甘露醇购于华仁药业(日照)有限公司;造影剂购于江苏恒瑞医药股份有限公司;ROS试剂盒,苯甲基磺酰氟,裂解液,bak兔多抗,caspase-3单克隆抗体,原位末端标记法(TdT-mediated dUTP Nick-End Labeling,TUNEL)试剂盒,PVDF膜,SDS-PAGE凝胶,预染彩虹maker,溴酚兰,QuickBlock封闭液,TBS-T,超敏ECL发光液,辣根过氧化物酶标记山羊抗兔IgG(H+L)均购于上海碧云天科技有限公司;苏木素,伊红染液购于珠海贝索生物技术有限公司;bcl-2兔多抗购于北京索莱宝科技有限公司;电泳槽购于北京君意东方电泳设备有限公司,型号:JV-ZY6;脱色摇床购于泰州诺米医疗科技有限公司,型号:NYC-80;多功能手术仪(双极电凝)购于武汉春光医疗美容仪器有限公司,型号:CHR-V;小动物麻醉机购于ABM;体温维持仪购于上海玉妍科学仪器有限公司,型号:XMTF-7000;离心机购于湖南安君研仪器有限公司,型号:A16K-R;酶标分析仪购于南京德铁实验设备有限公司,型号:HBS-1096B;荧光显镜购于广州市明美光电技术有限公司,型号:MF43。
1.3 动物实验
1.3.1 模型制备及验证 采用大鼠颈内动脉穿刺,输注甘露醇和造影剂的方法制备SAH致AKI模型。将大鼠置入预充七氟醚的麻醉箱进行麻醉诱导,待麻醉满意后仰卧位固定于铺有温毯的操作台上,设置肛温<38 ℃时温毯加热,3%~4%七氟醚麻醉维持。在模型制备前经鼠尾静脉采集血液测定血肌酐(serum creatinine,SCr)(0 h SCr),备皮、消毒、铺巾,正中切口,逐层分离,暴露左颈动脉,结扎离断颈外动脉分支并电凝止血。无损伤血管钳夹闭颈总动脉,颈内动脉。结扎颈外动脉远端并切开拉直,使得其与颈内动脉呈一直线。从颈外动脉将头端磨锐的尼龙线置入,直至有阻力感继续置入3 mm左右,刺破颈内动脉颅内段(20±2) mm,造成SAH,随后撤出尼龙线,结扎颈外动脉近心端,开放颈总动脉,缝合切口,并给予0.25%罗哌卡因局部封闭镇痛。随后从鼠尾静脉分别缓慢静注甘露醇9 g/kg,注入造影剂2 mL。对照组模型除不刺破大脑中动脉血管其余操作均相同。所有大鼠均回笼单独饲养。模型制备后24 h(24 h SCr)经鼠尾静脉采集血液测定SCr。24 h SCr>1.5×0 h SCr认为SAH致大鼠AKI模型制备成功。
1.3.2 实验分组 制备对照组12只,无死亡;制备SAH致AKI模型使用大鼠41只,死亡8只,SAH致AKI模型(24 h SCr>1.5×0 h SCr)成功24只,随机分为模型组(n=12)和氢气治疗组(n=12)。制备模型成功后24 h和48 h时,将氢气治疗组大鼠置入预充2.9%浓度氢气的麻醉诱导箱中2 h,将模型组和对照组大鼠置入预充空气的诱导箱2 h。
1.3.3 AKI血清学参数分析 各组大鼠在组织灌注前,从主动脉采集血样0.7~1.0 mL,测定BUN和SCr水平。
1.3.4 苏木精-伊红染色法(hematoxylin-eosin staining,HE)测定大鼠肾脏病理改变 模型制备成功后72 h,在6%七氟醚深度麻醉下,迅速去除胸骨打开胸腔,充分暴露心脏,于心尖处取血2 mL,随后使用连接输液器的穿刺针从心尖部位插入,向上进针到升主动脉并使用血管钳固定。剪开右心耳后使用生理盐水灌注,直至肝,肾和肺脏颜色转白,右心耳流出的液体清亮后,灌注10%甲醛250 mL,摘取右肾(n=6)。经固定、脱水、透明、浸腊、包埋后,切制5 μm石蜡切片。经二甲苯Ⅰ、Ⅱ分别浸泡10 min后,不同浓度梯度酒精(100%,90%,80%,70%)脱蜡,各2 min,随后置入苏木素染液中染色6 min,水化30 min后置入伊红染色液中染色3 min,水洗2 min后置入不同浓度梯度酒精(70%、80%、90%、100%)脱水,各30 s,再放入二甲苯Ⅰ、Ⅱ中分别透明10 min,滴加中性树胶后封片,观察。肾脏病理评分依据Paller评分法:即每高倍镜视野随机选择10个有病变的肾小管,按100个肾小管记分,肾小管明显扩张、细胞扁平记1分;刷状缘损1分,脱落记2分;肾小管管腔内有脱落的、坏死的细胞包括未成管型或细胞碎片记1分,管型记2分。
1.3.5 肾脏组织活性氧(reactive oxygen species,ROS)水平的测定 模型制备成功后72 h,在8%七氟醚麻醉下摘取右肾,将0.1 mL含1 mg肾脏皮质组织、2.9 mL ROS分析介质和15 nmol/L 2′,7′-二氯氟二乙酸混匀后,在37 ℃温箱中孵育15 min,酶标仪测定488 nm激发波长,525 nm发射波长下的荧光值。
1.3.6 蛋白印迹法测定大鼠肾脏组织B淋巴细胞瘤2 (B-cell lymphoma-2,bcl-2)、bcl-2相关蛋k(bcl-2 associated-k,bak)以及裂解的半胱氨酸蛋白酶3(cleaved caspase-3)蛋白表达 模型制备成功后72 h,每组取6只大鼠的肾脏组织100 mg(n=6)。充分剪碎、研磨组织,加入500 μL苯甲基磺酰氟和500 μL裂解液进行组织匀浆,在4 ℃,12 000 r/min,半径=8 cm条件下离心5 min,取上清采用二喹啉甲酸法测定蛋白含量。计算含30 μg蛋白的溶液体积即为上样量,加入上样缓冲液后于水中煮沸5 min至蛋白变性,加入10% SDS-PAGE凝胶上样孔中,恒压80 V电泳至预染彩虹预染蛋白分离清晰,溴酚兰即将跑出凝胶。随后采用80 V恒压湿转50 min,将蛋白转至聚偏二氟乙烯膜,随后移至含有封闭液的平皿中,室温下脱色摇床上摇动封闭1 h。TBS-T缓冲液洗涤聚偏二氟乙烯膜后,分别加入bcl-2(1∶1 000),bak(1∶1 000),caspase-3(1∶1 000)一抗,4 ℃过夜。TBS-T洗膜3次,每次5 min。加入辣根过氧化物酶标记山羊抗兔IgG(H+L)(1∶1 000)室温下孵育1 h。TBS-T缓冲液洗膜3次每次5 min。加入ECL发光液,在脱色摇床上反应5 min,发光仪显影。GAPDH作为内参蛋白,以目的蛋白与内参蛋白条带的灰度比值表示蛋白表达水平。
1.3.7 免疫荧光法测定大鼠肾脏细胞凋亡率 每组取6只大鼠的肾脏石蜡切片(n=6),在二甲苯Ⅰ、Ⅱ各浸泡10 min以及不同浓度梯度酒精(100%、90%、80%、70%)脱蜡后,置于装有PBS的染色缸中洗片3次,每次5 min。滴加20 μL不含DNase的蛋白酶K,于37 ℃温箱中孵育反应35 min,置于装有PBS的染色缸中洗片3次,每次5 min。随后加入TUNEL检测液,在37 ℃温箱中避光孵育60 min,置于装有PBS的染色缸中洗片3次,每次5 min,滴加含DAPI的封片液,盖玻片封片,荧光显微镜下进行观察。计算TUNEL/DAPI双阳性细胞数占总细胞的百分比即为细胞凋亡率,计数5个高倍视野取平均值。
1.4 细胞实验
1.4.1 肾小管上皮细胞的培养及分组 肾小管上皮细胞系NRK52E用含10%胎牛血清的DMEM培养基,于37 ℃、5% CO2恒温箱培养,每天换液1次,待细胞融和至90%,加入胰蛋白酶进行消化传代,取对数生长期细胞用于实验;模型组用300 μmol/L过氧化氢干预24 h建立氧化应激损伤模型,氢气治疗组在过氧化氢干预后24 h和48 h分别给予2.9%氢气处理2 h。
1.4.2 肾小管上皮细胞丙二醛(malonaldehyde,MDA)和超氧化物歧化酶(superoxide dismutase,SOD)测定 待细胞干预结束后取各组细胞培养基1 mL,按照试剂盒说明书要求处理样品,硫代巴比妥酸法检测MDA,黄嘌呤氧化法检测SOD,按照试剂盒步骤操作。每次设3个复孔,重复3次。
1.4.3 流式细胞仪检测肾小管上皮细胞凋亡水平 各组细胞干预后培养2 d,添加0.25%胰蛋白酶后收集各组细胞,用150 μL缓冲液将各组细胞悬浮,添加Annexin V-FITC约10 μL后,避光孵育15 min。添加5 μL PI均匀混合,继续添加150 μL缓冲液,立即用流式细胞仪检测细胞凋亡率,每次设3个复孔,重复3次。
1.4.4 四甲基偶氮唑蓝(Methyl Thiazolyl Tetrazolium,MTT)法检测细胞存活率 各组细胞接种于96孔板上,每孔避光加入5 g/L的MTT溶液20 μL,继续孵育 4 h。弃去培养液,每孔加入二甲基亚砜150 μL,于微量振荡器上水平缓慢振荡1 min,待充分结晶沉淀后,用酶联检测仪检测波长570 nm处的吸光度(optical delnsity,OD)值,计算存活率=实验组OD值/对照组OD值×100%。
1.5 统计学方法 应用SPSS 17.0统计软件分析数据。计量资料比较采用单因素方差分析和Tukey检验。P<0.05为差异有统计学意义。
2 结 果
2.1 氢气治疗对大鼠BUN和SCr水平的影响 模型组和氢气治疗组大鼠BUN和SCr水平高于对照组,氢气治疗组大鼠BUN和SCr水平低于模型组,差异有统计学意义(P<0.05)。见表1。
表1 各组大鼠BUN和SCr水平比较
Table 1 Comparison of blood urea nitrogen and
serum creatinine of rats in each group ![]()
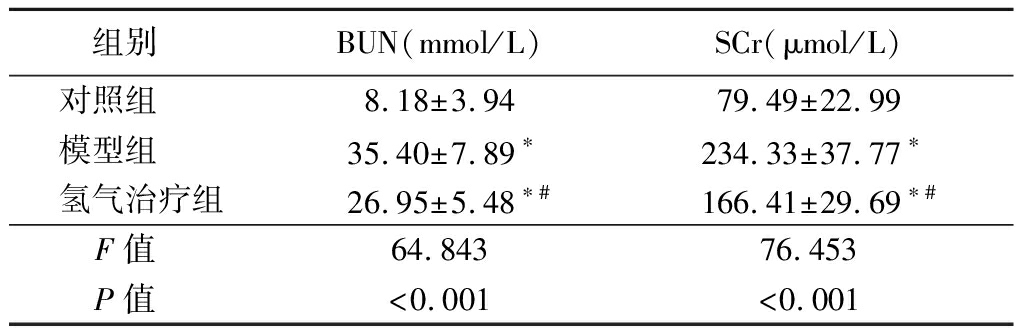
组别 BUN(mmol/L)SCr(μmol/L)对照组 8.18±3.9479.49±22.99模型组 35.40±7.89∗234.33±37.77∗氢气治疗组26.95±5.48∗#166.41±29.69∗#F值 64.84376.453P值 <0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与模型组比较(Tukey 检验)
2.2 氢气治疗对大鼠肾脏病理改变的影响 HE染色结果显示:对照组大鼠肾脏组织结构清晰,细胞排列紧密,细胞核及细胞质染色均匀,仅见少量肾小管上皮细胞刷状缘。模型组大鼠肾脏组织可见大量小管上皮细胞肿胀、颗粒及空泡变性;部分小管上皮细胞刷状缘、扁平、皱缩、脱落、核深染、裸露,严重部位见弥漫性细胞片状崩解、坏死,脱落,基底膜不完整;部分肾小管管腔扩张,管腔内可见上皮细胞碎片,肾间质水肿,充血及出血明显。氢气治疗组组织肾小管肿胀、充血、出血及细胞核脱落情况明显减轻(图1)。模型组和氢气治疗组大鼠肾小管损伤评分高于对照组,氢气治疗组大鼠肾小管损伤评分低于模型组,差异有统计学意义(P<0.05)。见表2。
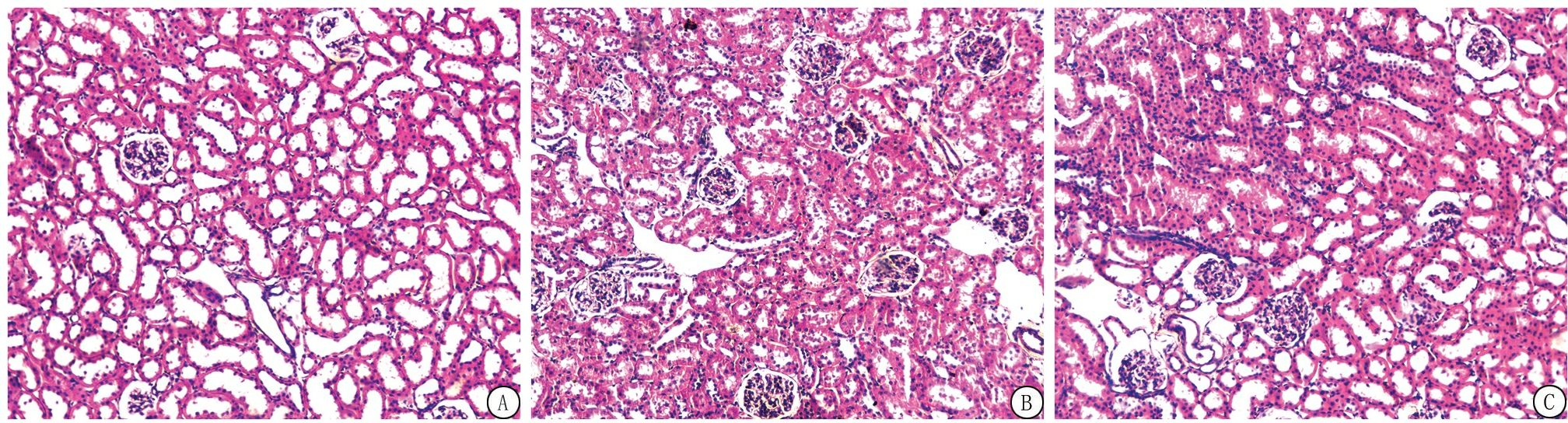
图1 各组大鼠肾脏组织HE染色(×100)
A.对照组;B.模型组;C.氢气治疗组
Figure 1 HE staining of renal tissue of rats in each group(× 100)
表2 各组大鼠肾小管损伤评分比较
Table 2 Comparison of renal tubular injury of
rats in each group ![]() 分)
分)
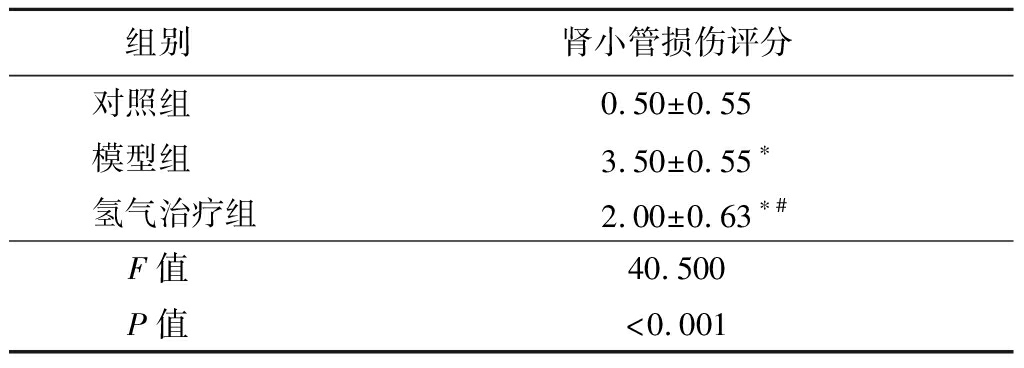
组别 肾小管损伤评分对照组 0.50±0.55模型组 3.50±0.55∗氢气治疗组2.00±0.63∗#F值 40.500P值 <0.001
*P值<0.05与对照组比较 #P值<0.05与模型组比较(Tukey 检验)
2.3 氢气治疗对大鼠肾脏组织中ROS水平的影响 模型组和氢气治疗组大鼠肾脏组织中ROS水平高于对照组,氢气治疗组大鼠肾脏组织中ROS水平低于模型组,差异有统计学意义(P<0.05)。见表3。
表3 各组大鼠肾脏组织ROS水平比较
Table 3 The content of ROS in the renal tissue of
rats in each group ![]()
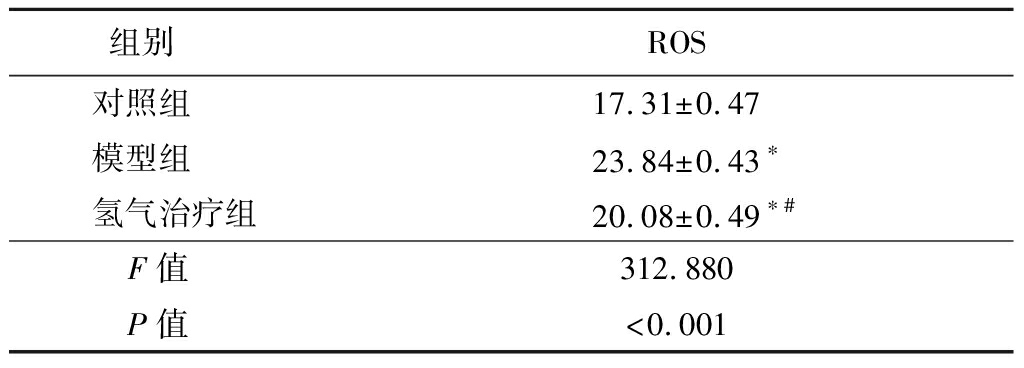
组别 ROS对照组 17.31±0.47模型组 23.84±0.43∗氢气治疗组20.08±0.49∗#F值 312.880P值 <0.001
*P值<0.05与对照组比较 #P值<0.05与模型组比较(Tukey 检验)
2.4 氢气治疗对大鼠肾脏组织中bcl-2、bak和cleaved caspase-3蛋白表达的影响 模型组和氢气治疗组大鼠肾脏组织bcl-2/bak蛋白比值低于对照组,cleaved caspase-3蛋白表达高于对照组;氢气治疗组大鼠肾脏组织bcl-2/bak蛋白比值高于模型组,cleaved caspase-3蛋白表达低于模型组,差异有统计学意义(P<0.05)。见图2,表4。
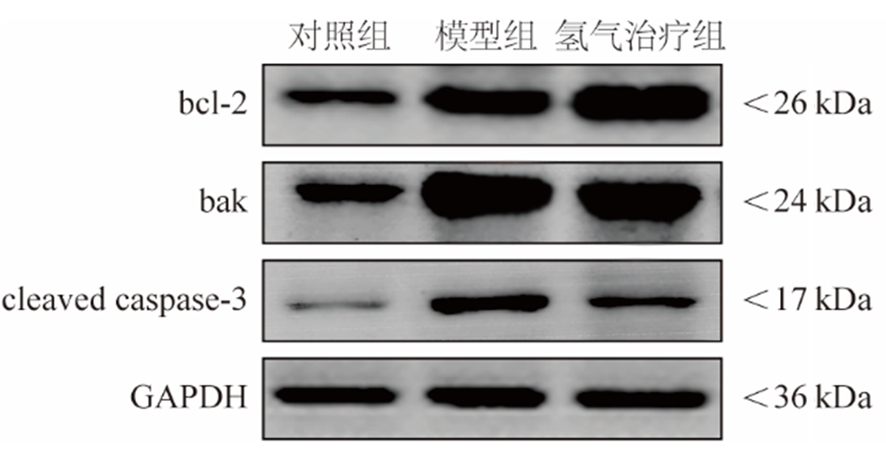
图2 各组大鼠肾脏组织bcl-2、bak、cleaved caspase-3和内参的蛋白印记
Figure 2 Western blots of bcl-2, bak, cleaved caspase-3 and GADPH in the renal tissue of rats in each group
表4 各组大鼠肾脏组织bcl-2/bak比值和cleaved
caspase-3蛋白表达比较
Table 4 Comparison of bcl-2/bak ratio and cleaved
caspase-3 expressions in the renal tissue of
rats in each group![]()
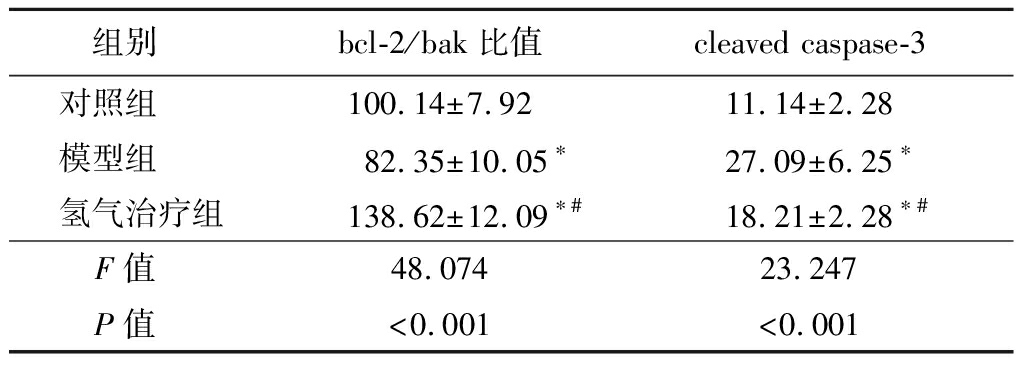
组别 bcl-2/bak比值cleaved caspase-3对照组 100.14±7.9211.14±2.28模型组 82.35±10.05∗27.09±6.25∗氢气治疗组138.62±12.09∗#18.21±2.28∗#F值 48.07423.247P值 <0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与模型组比较(Tukey 检验)
2.5 氢气治疗对大鼠肾脏细胞凋亡率的影响 模型组和氢气治疗组大鼠肾脏细胞凋亡率高于对照组,氢气治疗组大鼠肾脏细胞凋亡率低于模型组,差异有统计学意义(P<0.05)。见表5。
表5 各组大鼠肾脏细胞凋亡率比较
Table 5 Apoptotic rate of renal cells of rats in each group![]()
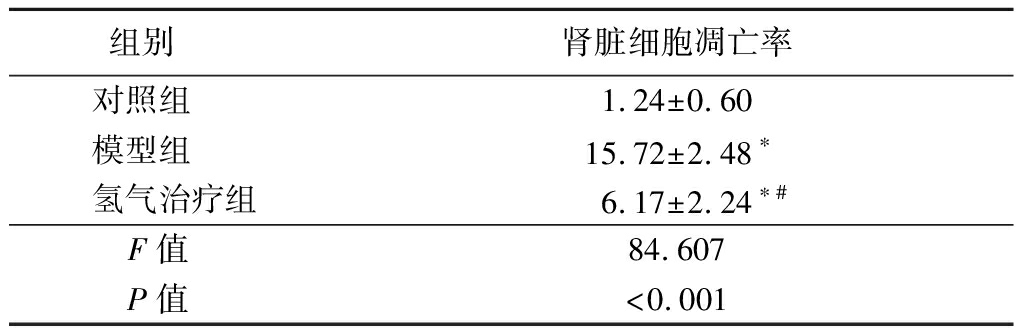
组别 肾脏细胞凋亡率对照组 1.24±0.60模型组 15.72±2.48∗氢气治疗组6.17±2.24∗#F值 84.607P值 <0.001
*P值<0.05与对照组比较 #P值<0.01与模型组比较(Tukey 检验)
2.6 氢气治疗对氧化应激肾小管上皮细胞MDA、SOD、存活率和凋亡率的影响 模型组和氢气治疗组肾小管上皮细胞MDA水平和凋亡率高于对照组,SOD水平和存活率低于对照组;氢气治疗组肾小管上皮细胞MDA和凋亡率低于模型组,SOD和存活率高于模型组,差异有统计学意义(P<0.05)。见表6。
表6 各组肾小管上皮细胞MDA、SOD、凋亡率和存活率比较
Table 6 Comparison of malondialdehyde,superoxide dismutase,apoptotic rate,and survival rate of tubular epithelial cells in each group![]()
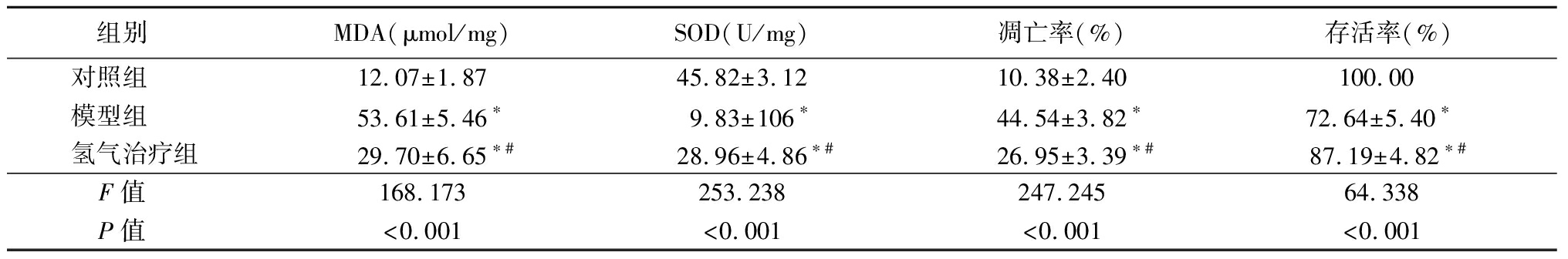
组别 MDA(μmol/mg)SOD(U/mg)凋亡率(%)存活率(%)对照组 12.07±1.8745.82±3.1210.38±2.40100.00 模型组 53.61±5.46∗9.83±106∗44.54±3.82∗72.64±5.40∗ 氢气治疗组29.70±6.65∗#28.96±4.86∗#26.95±3.39∗#87.19±4.82∗#F值 168.173253.238247.24564.338P值 <0.001<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与模型组比较(Tukey 检验)
3 讨 论
SAH是一种严重威胁人类生命健康的急危重症,其中85%出血原因为颅内动脉瘤破裂,临床上主要表现为颅内压升高、患者意识减退甚至消失[7]。目前认为早期脑损伤是SAH致死致残的主要原因,其病理生理机制主要包括动脉瘤破裂后的机械性损伤、颅内压升高、脑血流量降低、脑灌注压下降、大脑皮质广泛去极化、细胞凋亡和坏死自噬作用、离子稳态破坏、炎症反应、血脑屏障破坏等[8-10]。早期脑损伤在损伤神经功能的同时,也导致了全身应激反应、炎症反应以及神经体液改变等,进而导致心脏、肺、肾及胃肠道等远隔器官的损伤[11-12]。甘露醇作为降低颅内压既经济又有效的药物,常应用于SAH患者,造影剂是SAH介入诊断及治疗的必备药,两者的应用均可加重肾脏负担。本研究参考文献[13]通过颈外动脉置管,经颈内动脉刺破大脑中动脉,制备SAH模型,并在造模成功后经鼠尾静脉给予甘露醇及造影剂,模拟了SAH患者的入院诊治过程,将24 h SCr高于1.5倍基础 SCr定为AKI。本研究结果表明,SAH大鼠应用造影剂及甘露醇后,肾功能受损,AKI发生率约为70%,提示SAH诱发的大鼠AKI模型建立成功。
既往研究表明,SAH早期诱发的儿茶酚胺大量释放,导致外周器官缺血缺氧,并诱发多种炎性因子活化;SAH可诱发脑血管痉挛,缺氧后的脑组织造成线粒体能量耗竭,多种氧离子及过氧化氢生成,血脑屏障完整性下降,炎性因子及氧自由基释放入血,进一步加重全身炎性反应[14]。肾脏作为血流量最大的外周远隔器官之一,SAH诱发的AKI是临床中最为常见的并发症之一,已有研究表明,SAH诱发的AKI可能与小管上皮细胞氧化应激、炎症反应,进一步诱导肾小管上皮细胞凋亡反应有关[15]。线粒体作为氧化应激反应的主要靶点,缺血再灌注损伤可导致ROS水平急剧升高,诱导凋亡因子bak活化,导致线粒体外膜通透性增强,进而诱发细胞凋亡;bcl-2作为最重要的抗凋亡因子,通过抑制bak活化,保持线粒体膜完整性,抑制细胞凋亡[16]。既往研究表明,bcl-2/bak比值的改变可调控细胞凋亡,bcl-2/bak比值下调,表明细胞正在发生凋亡[17]。caspase-3作为凋亡级联反应的最后一级,活化后裂解为cleaved caspase-3,通过进入细胞核切割DNA,诱发细胞凋亡[17]。本研究结果显示,SAH后大鼠肾脏组织ROS水平升高,bcl-2/bak比值下调,cleaved caspase-3表达上调,肾脏组织细胞凋亡率升高,SCr、BUN及肾损伤评分升高,提示了SAH相关性AKI与氧化应激后诱发肾小管上皮细胞凋亡相关。
氢气作为一种无色无味不易溶于水的气体,近年来被发现其具有强大的抗氧化作用;与其它吸入性治疗药物比较,具有易扩散、起效快、无明显不良反应等优点[18]。Jiang等[19]报道,吸入2%氢气能选择性清除·OH和ONOO-,减轻脑缺血再灌注引起的氧化应激损伤;Kumagai等[20]研究表明,氢气通过抑制线粒体生成过氧化物,显著减轻SAH诱发的神经细胞凋亡。本研究通过模拟SAH诱发的AKI型,在SAH后24 h、48 h时吸入2.9%氢气2 h进行治疗,结果显示,氢气治疗组SCr、BUN及损伤评分减低,表明氢气对SAH诱发的AKI具有保护作用;氢气显著减少了肾组织中的ROS含量,bcl-2/bak比值上调,肾脏细胞凋亡率降低,cleaved caspase-3表达下调,表明,氢气对SAH诱发AKI的保护作用与减轻氧化应激,抑制肾细胞凋亡相关。
为了进一步证实氢气对SAH诱发AKI的保护作用,本研究采用过氧化氢处理肾小管上皮细胞的体外模型,结果显示,与单纯过氧化氢处理肾小管上皮细胞比较,氢气可显著降低过氧化氢处理的肾小管上皮细胞中的MDA水平,并增加SOD水平,同时肾小管上皮细胞凋亡率显著降低。表明氢气减轻SAH诱发AKI的机制与其抑制氧化应激减少肾小管上皮细胞凋亡相关,与Ming等[21]的研究结果一致。
综上所述,氢气对SAH致AKI大鼠具有保护作用,其机制与氢气能减少ROS产生,减少氧化应激,上调bcl-2/bak比值从而抑制肾脏细胞凋亡有关。
[1] Gritti P,Akeju O,Lorini FL,et al. A narrative review of adherence to subarachnoid hemorrhage guidelines[J]. J Neurosurg Anesthesiol,2018,30(3):203-216.
[2] Pan CY,Tian M,Zhang LL,et al. lncRNA signature for predicting cerebral vasospasm in patients with SAH:implications for precision neurosurgery[J]. Mol Ther Nucleic Acids,2020,21:983-990.
[3] Eagles ME,Powell MF,Ayling OGS,et al. Acute kidney injury after aneurysmal subarachnoid hemorrhage and its effect on patient outcome:an exploratory analysis[J]. J Neurosurg,2019,12:1-8.
[4] Tamura T,Suzuki M,Hayashida K,et al. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome[J]. J Clin Biochem Nutr,2020,67(2):214-221.
[5] 谢克亮,王瑶琪,于泳浩,等.氢气在麻醉学领域的应用[J].中华麻醉学杂志,2019,39(5):513-516.
[6] Camara R,Matei N,Camara J,et al. Hydrogen gas therapy improves survival rate and neurological deficits in subarachnoid hemorrhage rats:a pilot study[J]. Med Gas Res,2019,9(2):74-79.
[7] 秦超,刘竞丽.蛛网膜下腔出血的诊断与治疗[J].中华神经科杂志,2020,53(10):814-818.
[8] Zhou J,Gu L,Peng JH,et al. Expression of translocator protein in early brain injury after subarachnoid hemorrhage in mice[J]. Sichuan Da Xue Xue Bao Yi Xue Ban,2019,50(4):500-505.
[9] Zhang HB,Tu XK,Song SW,et al. Baicalin reduces early brain injury after subarachnoid hemorrhage in rats[J]. Chin J Integr Med,2020,26(7):510-518.
[10] Saand AR,Yu F,Chen J,et al. Systemic inflammation in hemorrhagic strokes-a novel neurological sign and therapeutic target?[J]. J Cereb Blood Flow Metab,2019,39(6):959-988.
[11] Yang S,Chen X,Li S,et al. Melatonin treatment regulates SIRT3 expression in early brain injury(EBI) due to reactive oxygen species(ROS) in a mouse model of subarachnoid hemorrhage(SAH)[J]. Med Sci Monit,2018,24:3804-3814.
[12] Bansal KK,Singh PK. “Transient reactionary physiological asystole”-TRAP phenomenon? Cause of fall or loss of consciousness after sub arachnoid hemorrhage(SAH)[J]. Br J Neurosurg,2018,32(3):302.
[13] Thompson JW,Elwardany O,McCarthy DJ,et al. In vivo cerebral aneurysm models[J]. Neurosurg Focus,2019,47(1):E20.
[14] Fumoto T,Naraoka M,Katagai T,et al. The role of oxidative stress in microvascular disturbances after experimental subarachnoid hemorrhage[J]. Transl Stroke Res,2019,10(6):684-694.
[15] Tujjar O,Belloni I,Hougardy JM,et al. Acute kidney injury after subarachnoid hemorrhage[J]. J Neurosurg Anesthesiol,2017,29(2):140-149.
[16] Rahmani M,Nkwocha J,Hawkins E,et al. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells[J]. Cancer Res,2018,78(11):3075-3086.
[17] Pe a-Blanco A,García-S
a-Blanco A,García-S ez AJ. Bax,bak and beyond-mitochondrial performance in apoptosis[J]. FEBS J,2018,285(3):416-431.
ez AJ. Bax,bak and beyond-mitochondrial performance in apoptosis[J]. FEBS J,2018,285(3):416-431.
[18] Tao G,Song G,Qin S. Molecular hydrogen:current knowledge on mechanism in alleviating free radical damage and diseases[J]. Acta Biochim Biophys Sin(Shanghai),2019,51(12):1189-1197.
[19] Jiang Z,Alamuri TT,Muir ER,et al. Longitudinal multiparametric MRI study of hydrogen-enriched water with minocycline combination therapy in experimental ischemic stroke in rats[J]. Brain Res,2020,1748:147122.
[20] Kumagai K,Toyooka T,Takeuchi S,et al. Hydrogen gas inhalation improves delayed brain injury by alleviating early brain injury after experimental subarachnoid hemorrhage[J]. Sci Rep,2020,10(1):12319.
[21] Ming Y,Ma QH,Han XL,et al. Molecular hydrogen improves type 2 diabetes through inhibiting oxidative stress[J]. Exp Ther Med,2020,20(1):359-366.