近年来,慢性肾脏病(chronic kidney disease,CKD)发病率在全球范围内呈逐年增高态势,而我国成年人CKD发病率高达10.8%~13.4%,已成为了当今世纪人类面临的重大公共健康问题[1]。而CKD矿物质和骨异常(CKD-mineral and bone disorder,CKD-MBD)是诱发CKD患者致残、致死等不良结局的关键因素,以钙磷代谢异常、骨骼成分和结构改变、继发性甲状旁腺功能亢进、血管和软组织钙化为主要临床表现,在CKD早期即可出现,随着CKD病情进展而逐渐加重,不仅会增加心血管不良事件发生风险,还与CKD患者心血管钙化、骨折等有关[2]。近年来有研究发现,骨硬化蛋白(sclerostin,SOST)作为骨重建过程中的关键蛋白,由位于人染色体的17q12~q21的SOST基因编码转录形成,可经抑制骨代谢调节信号通路(Wnt通路、转化生长因子β/骨形态发生蛋白通路)在骨形成中发挥重要的负性调控作用,抑制成骨细胞发育及骨形成,可能参与CKD-MBD的发生及发展,但关于其与CKD 3~5期患者血钙(calcium,Ca)、血磷(phosphorus,P)、钙磷乘积(calcium phosphorus product,Ca·P)、全段甲状旁腺素(intact parathyroid hormone,iPTH)、25-羟维生素D3[25-hydroxyvitamin D3,25-(OH)D3]等矿物质骨代谢指标的相关性鲜有报道[3-4]。因此,本研究旨在通过探讨CKD患者血清SOST水平与CKD-MBD的相关性,为CKD-MBD防治提供参考,现报告如下。
1 资料与方法
1.1 一般资料 选取2017年2月—2019年12月蚌埠医学院第二附属医院收治的CKD 3~5期患者120例,其中CKD 3期、CKD 4期、CKD 5期各40例。另选取同期年龄、性别相匹配的健康体检者40例为对照组,未发现心、肾、骨骼及内分泌等系统病变,无CKD及骨代谢异常。4组性别、年龄、体重指数(body mass index,BMI)、原发疾病比较差异无统计学意义(P>0.05),具有可比性。见表1。
表1 4组一般资料比较
Table 1 Comparison of general data between four groups (n=40)
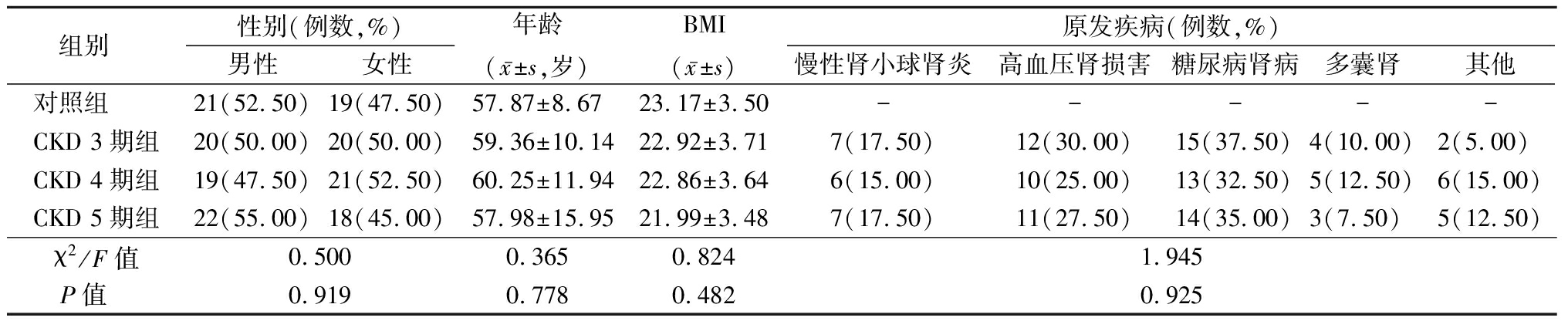
组别 性别(例数,%)男性女性年龄(x-±s,岁)BMI(x-±s)原发疾病(例数,%)慢性肾小球肾炎高血压肾损害糖尿病肾病多囊肾其他对照组 21(52.50)19(47.50)57.87±8.6723.17±3.50-----CKD 3期组20(50.00)20(50.00)59.36±10.1422.92±3.717(17.50)12(30.00)15(37.50)4(10.00)2(5.00) CKD 4期组19(47.50)21(52.50)60.25±11.9422.86±3.646(15.00)10(25.00)13(32.50)5(12.50)6(15.00)CKD 5期组22(55.00)18(45.00)57.98±15.9521.99±3.487(17.50)11(27.50)14(35.00)3(7.50) 5(12.50)χ2/F值0.5000.3650.8241.945P值 0.9190.7780.4820.925
本研究经医院医学伦理委员会审批通过,所有受试者者均知情同意并签署知情同意书。
1.2 纳入标准和排除标准 纳入标准:①CKD符合《慢性肾脏病筛查诊断及防治指南》中CKD诊断标准[5],根据估算肾小球滤过率(estimated glomerular filtration rate,eGFR)分期,eGFR 30~59 mL·min-1·1.73 m-2判断为CKD 3期,eGFR 15~29 mL·min-1·1.73 m-2判断为CKD 4期,eGFR<15 mL·min-1·1.73 m-2判断为CKD 5期;②CKD病史超过12个月;③未接受透析治疗;④临床资料完整。排除标准:①合并自身免疫性肾损伤、急性肾损伤、严重肝病史;②短期内存在隐形或显性感染;③骨折、原发骨代谢疾病;④原发性甲状旁腺功能亢进;⑤血液系统疾病;⑥良性或恶性肿瘤;⑦近期接受过激素及免疫抑制治疗,或近30 d内使用药物纠正钙磷代谢或应用过维生素D制剂;⑧妊娠期或哺乳期妇女;⑨患有精神疾病无法配合本研究者。
1.3 研究方法 所有研究对象入院后详细采集病史资料,包括性别、年龄、BMI、原发疾病。对照组于体检当日清晨抽取空腹静脉血5 mL,CKD 3~5期患者于入院次日清晨抽取空腹静脉血5 mL,均于室温下静置60 min,3 000 r/min离心10 min,待血清分离后置于-70 ℃冰箱中保存备测。①利用肾脏病饮食改良(modification of diet in renal disease,MDRD)简化公式计算出eGFR,其中eGFR=30 849.2×(血肌酐)-1.154×(年龄)-0.203,若为女性则再乘以0.742。②采用贝克曼AU5800全自动生化仪检测常规生化指标,其中酶动力法测定血肌酐(serum creatinine,SCr),尿素酶速率法测定血尿素氮(blood urea nitrogen,BUN);偶氮胂Ⅲ法检测Ca,磷钼酸还原法检测P,并计算Ca·P;比色法检测碱性磷酸酶(alkaline phosphatase,AKP),散射速率比浊法检测白蛋白(albumin,ALB),比色法测定血红蛋白(hemoglobin,Hb)。③采用酶联免疫吸附法检测SOST,同位素化学发光法测定iPTH(iPTH由合肥金域检验中心检测),试剂盒均由上海生物科技有限公司生产。④采用Cobas e601型全自动电化学发光免疫分析仪(罗氏公司)检测25-(OH)D3,试剂盒由上海雅吉生物科技有限公司生产。⑤采用XR-46双光能X线骨密度检测仪(美国Norland公司)检测患者腰椎的骨密度(bone density,BMD)。
1.4 统计学方法 应用SPSS 22.0统计软件分析数据。计量资料比较采用单因素方差分析和SNK-q检验;计数资料比较采用χ2检验;相关性分析采用Pearson法。P<0.05为差异有统计学意义。
2 结 果
2.1 4组eGFR、SCr、BUN、Hb水平比较 CKD 3期组、CKD 4期组和CKD 5期组eGFR、Hb显著低于对照组,SCr、BUN显著高于对照组,差异有统计学意义(P<0.05);CKD 4期组和CKD 5期组eGFR、Hb显著低于CKD 3期组,SCr、BUN显著升高于CKD 3期组,差异有统计学意义(P<0.05);CKD 5期组eGFR、Hb显著低于CKD 4期组,SCr、BUN显著高于CKD 4期组,差异有统计学意义(P<0.05),见表2。
表2 4组eGFR、SCr、BUN、Hb水平比较
Table 2 Comparison of eGFR, SCr, BUN and Hb levels among four groups![]()
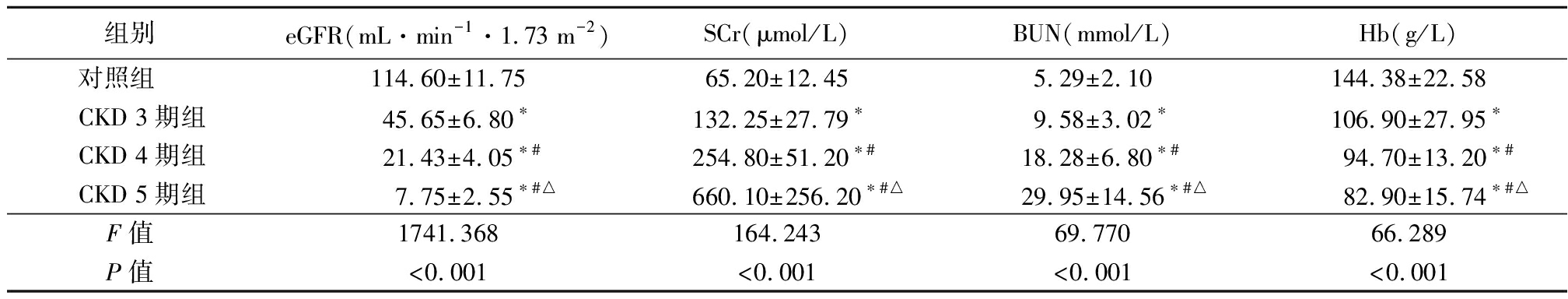
组别 eGFR(mL·min-1·1.73 m-2)SCr(μmol/L)BUN(mmol/L)Hb(g/L)对照组 114.60±11.7565.20±12.455.29±2.10144.38±22.58CKD 3期组45.65±6.80∗132.25±27.79∗9.58±3.02∗106.90±27.95∗CKD 4期组21.43±4.05∗#254.80±51.20∗#18.28±6.80∗#94.70±13.20∗#CKD 5期组7.75±2.55∗#△660.10±256.20∗#△29.95±14.56∗#△82.90±15.74∗#△F值 1741.368164.24369.77066.289P值 <0.001<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与CKD 3期组比较 △P值<0.05与CKD 4期组比较(SNK-q检验)
2.2 4组Ca、P、Ca·P、AKP、ALB、BMD水平比较 与对照组比较,CKD 3期组Ca、ALB、BMD显著低于对照组,CKD 4期组Ca、ALB、BMD显著低于对照组,P、Ca·P显著高于对照组,CKD 5期组Ca、ALB、BMD显著低于对照组,P、Ca·P、AKP显著高于对照组,差异有统计学意义(P<0.05);CKD 4期组Ca、BMD显著低于CKD 3期组,P、Ca·P显著高于CKD 3期组,CKD 5期组Ca、BMD显著低于CKD 3期组,P、Ca·P、AKP显著高于CKD 3期组,差异有统计学意义(P<0.05);CKD 5期组Ca、BMD显著低于CKD 4期组,P、Ca·P、AKP显著高于CKD 4期组,差异有统计学意义(P<0.05),见表3。
表3 4组Ca、P、Ca·P、AKP、ALB、BMD水平比较
Table 3 Comparison of Ca, P, CA·P, AKP, ALB, and BMD levels among four groups![]()
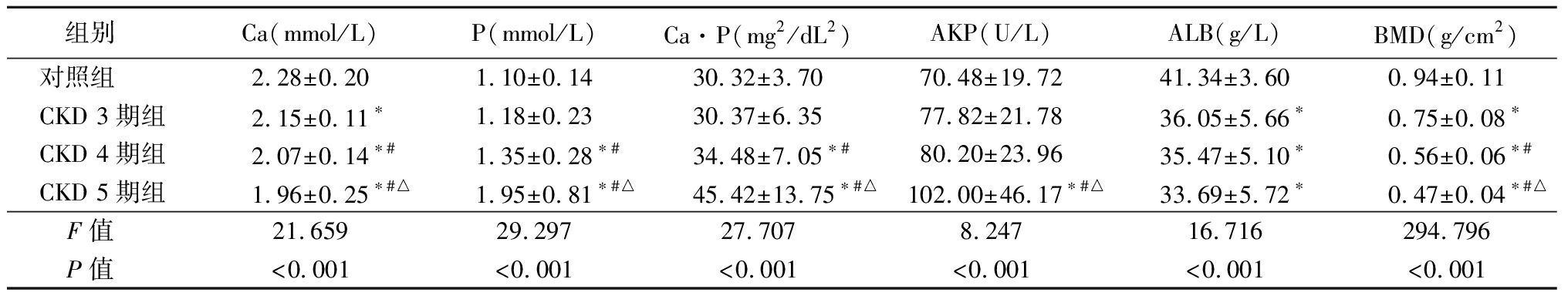
组别 Ca(mmol/L)P(mmol/L)Ca·P(mg2/dL2)AKP(U/L)ALB(g/L)BMD(g/cm2)对照组 2.28±0.201.10±0.1430.32±3.7070.48±19.7241.34±3.600.94±0.11CKD 3期组2.15±0.11∗1.18±0.2330.37±6.3577.82±21.7836.05±5.66∗0.75±0.08∗CKD 4期组2.07±0.14∗#1.35±0.28∗#34.48±7.05∗#80.20±23.9635.47±5.10∗0.56±0.06∗#CKD 5期组1.96±0.25∗#△1.95±0.81∗#△45.42±13.75∗#△102.00±46.17∗#△33.69±5.72∗0.47±0.04∗#△F值 21.65929.29727.7078.24716.716294.796P值 <0.001<0.001<0.001<0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与CKD 3期组比较 △P值<0.05与CKD 4期组比较(SNK-q检验)
2.3 4组SOST、iPTH、25-(OH)D3水平比较 CKD 3期组、CKD 4期组和CKD 5期组SOST、iPTH显著高于对照组,25-(OH)D3显著低于对照组,差异有统计学意义(P<0.05);CKD 4期组和CKD 5期组SOST、iPTH显著高于CKD 3期组,25-(OH)D3显著低于CKD 3期组,差异有统计学意义(P<0.05);CKD 5期组SOST、iPTH显著高于CKD 4期组,25-(OH)D3显著低于CKD 4期组,差异有统计学意义(P<0.05),见表4。
表4 4组SOST、iPTH、25-(OH)D3水平比较
Table 4 Comparison of SOST, iPTH and 25-(OH)D3
levels among four groups![]()
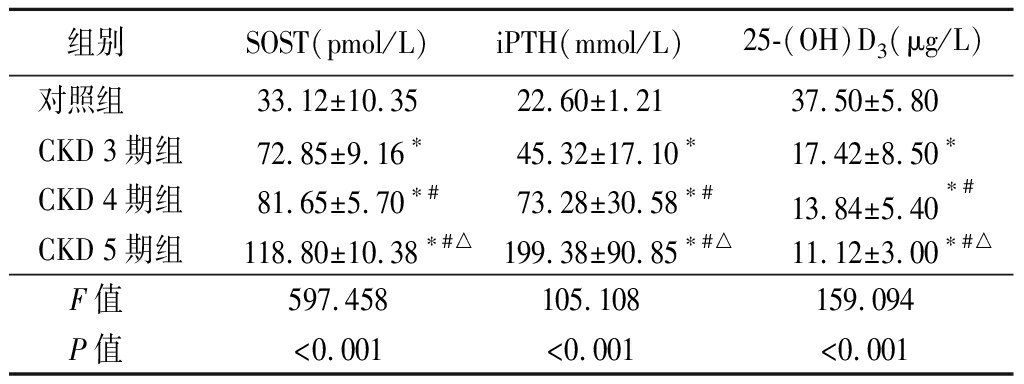
组别 SOST(pmol/L)iPTH(mmol/L)25-(OH)D3(μg/L)对照组 33.12±10.3522.60±1.2137.50±5.80CKD 3期组72.85±9.16∗45.32±17.10∗17.42±8.50∗CKD 4期组81.65±5.70∗#73.28±30.58∗#13.84±5.40∗#CKD 5期组118.80±10.38∗#△199.38±90.85∗#△11.12±3.00∗#△F值 597.458105.108159.094P值 <0.001<0.001<0.001
*P值<0.05与对照组比较 #P值<0.05与CKD 3期组比较 △P值<0.05与CKD 4期组比较(SNK-q检验)
2.4 CKD患者SOST与各实验室指标的相关性分析 经Pearson分析显示,CKD 3~5期患者SOST与eGFR、Hb、Ca、BMD、25-(OH)D3呈负相关(P<0.05),与SCr、P、Ca·P、iPTH呈正相关(P<0.05),与BUN、AKP、ALB无明显相关性(P>0.05),见表5。
表5 CKD患者SOST与各实验室指标的相关性分析
Table 5 Correlation analysis of serum SOST level and
laboratory indexes in patients with CKD
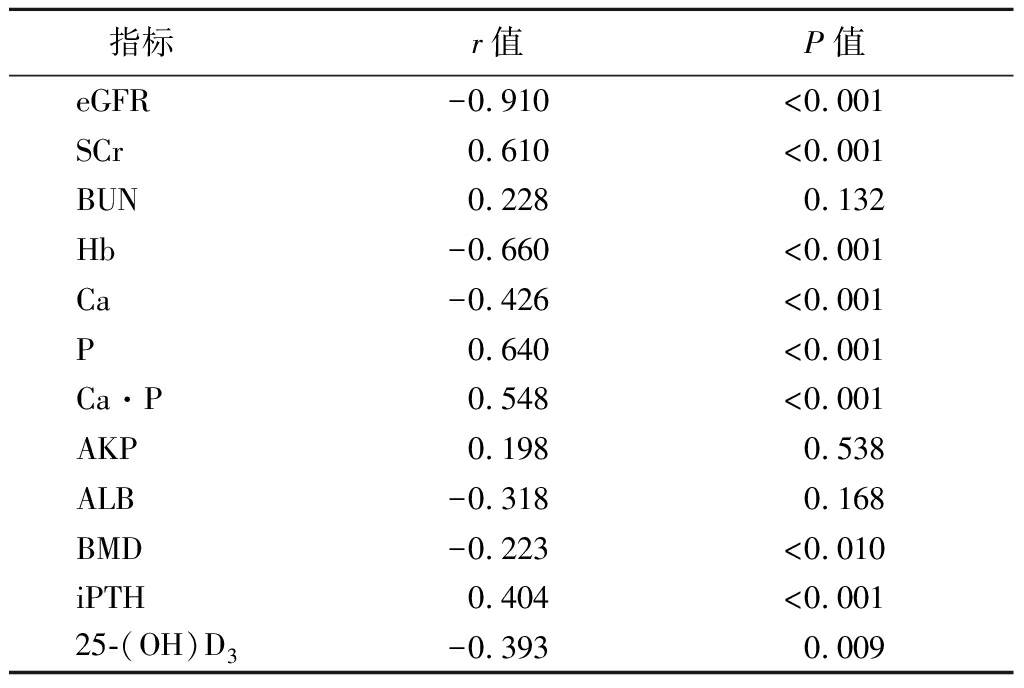
指标r值P值eGFR-0.910<0.001SCr0.610<0.001BUN0.2280.132Hb-0.660<0.001Ca-0.426<0.001P0.640<0.001Ca·P0.548<0.001AKP0.1980.538ALB-0.3180.168BMD-0.223<0.010iPTH0.404<0.00125-(OH)D3-0.3930.009
3 讨 论
CKD-MBD是指CKD患者系统性矿物质和骨代谢紊乱,早期(CKD 1~2期)常缺乏典型临床症状,中后期(CKD 3~5期)往往以生化指标异常(如Ca、P、iPTH、维生素D代谢紊乱)、血管或其他软组织异位钙化、肾性骨病为主要表现,与CKD患者不良心血管事件和病死率增加有关[6-7]。CKD-MBD发病机制复杂,主要与钙磷代谢紊乱与继发性甲状旁腺功能亢进、维生素D缺乏与肾性骨病、异位钙化有关,但P、iPTH等经典骨代谢标志物通常在CKD后期才出现显著改变[8]。近年来有报道表明,Wnt/β-catenin信号转导通路激活与CKD进展密切相关,而SOST作为Wnt/β-catenin信号转导通路的抑制剂之一,可能与CKD-MBD的发病有关[9]。本研究旨在探讨SOST与CKD-MBD的相关性,以期为CKD-MBD发病机制及治疗提供新的思路。
SOST属“胱氨酸结”分泌型蛋白DAN家族成员,主要由骨细胞分泌,并可表达于组织器官中,常通过Wnt/β-catenin信号通路、转化生长因子β/骨形态发生蛋白通路参与抑制骨形成过程,经改善成骨细胞分化及特异基因表达、调节骨基质形成及矿化等环节影响成骨作用[10]。Hamada-Ode等[11]报道,CKD患者血清SOST水平自CKD 3期开始逐渐升高,CKD 5期患者血清SOST水平明显高于CKD 3~4期患者,为正常对照组的2~4倍。Pietrzyk等[12]也证实,血清SOST水平自CKD 3期开始升高,且随着临床分期逐渐增加而不断升高。本研究结果显示,随着临床分期的增加(肾功能逐渐减退,即SCr逐步升高、eGFR逐渐降低),CKD 3~5期患者血清SOST水平逐步升高,推测可能与患者骨量减少和eGFR降低有关。进一步分析发现,CKD 3~5期患者SOST与eGFR呈负相关,与SCr呈正相关,证实了上述结论。推测其可能原因:①随着病情进展,eGFR逐渐降低,而在同等条件下SOST清除减少,致使血清浓度逐渐升高;②SOST分子量偏低,在尿路排泄方面与α1-微球蛋白相关,随着肾功能的逐渐减退,eGFR逐渐降低,尿中SOST逐渐增高,提示血清SOST水平升高与生成增多有关;③CKD患者骨量减少。但目前该机制尚不明确,有待今后进一步研究。
本研究结果显示,CKD 3期时出现Ca、25-(OH)D3降低,iPTH升高,CKD 4期时才出现P升高,且与对照组差异有统计学意义;Ca·P升高出现在CKD 4期,与P升高同期,但稍晚于Ca降低,基本相符指南[13]中提出的钙磷代谢紊乱和维生素D代谢紊乱出现时间。而进一步分析发现,CKD 3~5期患者SOST与Ca、25-(OH)D3呈负相关,与P、Ca·P、iPTH呈正相关,与既往报道[14-16]相符。推测原因,CKD 3期患者已出现Ca降低及25-(OH)D3减少,致使骨量减少,从而刺激血清SOST水平升高;而P、Ca·P升高是血管内膜钙化的影响因素,基于此刺激下患者血管壁中血管平滑肌细胞在核心结合因子影响下上调钙化核心因素(如AKP、非胶原蛋白等),促使血管平滑肌细胞转分化,致使CKD患者动脉中膜钙化,引起心脏瓣膜、软组织等器官钙化,最终影响血清SOST表达。此外,SOST通过抑制肾脏中1-α羟化酶细胞色素P450表达、上调成纤维细胞生长因子23水平等机制直接或间接抑制1,25-(OH)2D3形成,致使上游25-(OH)D3堆积,但其作用弱于对24,25-(OH)2D3生成的刺激作用,故致使CKD 3~5期患者25-(OH)D3呈逐渐降低趋势。而随着CKD患者病程进展,低Ca、高P刺激iPTH合成及分泌,诱导钙敏感受体等表达下调,促使iPTH生成增加;而iPTH对骨的影响有部分是经抑制SOST表达来介导的。推测可能是CKD疾病致使骨骼对iPTH刺激产生抵抗,导致iPTH信号活动减少,最终增加SOST产生;另外,考虑iPTH和SOST主要决定因素是肾功能状态,故推测也可能与此相关,今后仍需深入验证。此外,CKD 3~5期患者Hb水平随临床分期的增加而逐渐降低,与患者肾脏分泌促红细胞生成素降低,以及体内代谢毒物如SCr、BUN及其他类似代谢产物大量蓄积有关;而Hb降低会引起25-(OH)D3降低,又因CKD患者SOST与25-(OH)D3呈负相关,故推测Hb也与SOST存在相关性,具体机制仍需今后深入探究。
综上所述,CKD 3~5期患者血清SOST水平随着肾功能的降低而逐渐升高,并与Ca、P、Ca·P、iPTH等经典骨代谢标记物存在明显相关性,表明血清SOST有可能作为提示CKD-MBD的生物标记物。但本研究仍存在一定局限,未对不同骨硬化蛋白水平对矿物质、血管钙化及骨代谢的影响展开探讨,期待开展样本量更大的横向研究,故今后仍需深入调查研究。
[1] 赵璐,梅长林,邬碧波,等.上海市静安区慢性肾脏病高危人群社区筛查结果分析[J].中华肾脏病杂志,2020,36(1):1-5.
[2] 张睿,张艾佳.维持性血液透析患者慢性肾脏病-矿物质及骨代谢异常[J].中国老年学杂志,2019,39(17):4281-4283.
[3] 张洋洋,陈宇,杨佩钿,等.维持性血液透析患者血管钙化和血清骨硬化蛋白的相关因素[J].实用医学杂志,2018,34(23):3917-3920.
[4] 王雪,崔燕,赵永利,等.骨硬化蛋白在慢性肾脏病的研究进展[J].广东医学,2017,38(2):322-324.
[5] 上海慢性肾脏病早发现及规范化诊治与示范项目专家组.慢性肾脏病筛查诊断及防治指南[J].中国实用内科杂志,2017,37(1):28-34.
[6] Lunyera J,Scialla JJ. Update on chronic kidney disease mineral and bone disorder in cardiovascular disease[J]. Semin Nephrol,2018,38(6):542-558.
[7] 常立欣,张东雪.慢性肾脏病不同分期矿物质异常的横断面研究[J].河北医科大学学报,2018,39(1):97-100.
[8] Covic A,Vervloet M,Massy ZA,et al. Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population[J]. Lancet Diabetes Endocrinol,2018,6(4):319-331.
[9] 彭琼瑶,刘玲.骨硬化蛋白在慢性肾脏病血管钙化中的研究进展[J].临床肾脏病杂志,2019,19(3):218-222.
[10] 戴璇,李月红,姜埃利,等.骨硬化蛋白在慢性肾脏病发病及血管钙化中作用的研究进展[J].山东医药,2019,59(32):96-99.
[11] Hamada-Ode K,Taniguchi Y,Shimamura Y,et al. Serum dickkopf-related protein 1 and sclerostin may predict the progression of chronic kidney disease in Japanese patients[J]. Nephrol Dial Transplant,2019,34(8):1426-1427.
[12] Pietrzyk B,Wyskida K,Ficek J,et al. Relationship between plasma levels of sclerostin,calcium-phosphate disturbances,established markers of bone turnover,and inflammation in haemodialysis patients[J]. Int Urol Nephrol,2019,51(3):519-526.
[13] Ketteler M,Block GA,Evenepoel P,et al. Diagnosis,evaluation,prevention,and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the kidney disease: improving global outcomes 2017 clinical practice guideline update[J]. Ann Intern Med,2018,168(6):422-430.
[14] 付洁琼,李英.慢性肾脏病-矿物质与骨异常相关新型生物标志物的研究进展[J].中国中西医结合肾病杂志,2018,19(5):463-465.
[15] Lv W,Guan L,Zhang Y,et al. Sclerostin as a new key factor in vascular calcification in chronic kidney disease stages 3 and 4[J]. Int Urol Nephrol,2016,48(12):2043-2050.
[16] Paquot F,Delanaye P,Warling X,et al. Variations of sclerostin with other bone biomarkers over a one-year period in hemodialysis patients[J]. Clin Chim Acta,2018,486(1):183-184.