肝癌是常见的消化道恶性肿瘤之一[1-5],其主要治疗方式为手术切除和肝移植,但其预后较差,5年生存率只有20%~50%[6-7]。寻找肝癌相关的肿瘤标志物有助于改善患者预后。微小核醣核酸(MicroRNAs,miRNAs)是一种短链非编码RNAs,其与肝癌的发生和发展密切相关,是肝癌肿瘤标志物的合适选择[8-12]。研究显示, miR-18B-5p在肝癌细胞中有不同程度的表达,可以成为新的肝癌肿瘤标志物[13]。本研究旨在探讨miR-18B-5p和肝癌患者临床病理因素的关系及其作用机制,报告如下。
1 资 料 与 方 法
1.1 一般资料 选取2012年1月1日—2018年12月31日于河北医科大学第四医院进行手术治疗的原发性肝癌患者329例,随访至2020年5月31日,资料完整者321例,随访率为97.6%。其中存活84例,死于肝癌237例。随访时间2~103个月,平均(31.16±25.24)个月。其中,男性213例,女性108例;年龄41~73岁,平均(56.26±7.99)岁。
本研究经过医院伦理委员会批准,所有患者及家属均知情同意且签署知情同意书。
1.2 临床随访研究 出院后每6个月进行一次标准化随访。所有患者从诊断到死亡或最后一次随访至少12个月。生存期从患者入院时间开始计算,到患者死亡或最后随访时间为止点。
1.3 细胞系来源 实验室HepG2、Bel-7402、MHCC97、Hep3B细胞系购自南京凯基生物制品有限公司。采用10%胎牛血清(fetal bovine serum,FBS)(Gibco,美国)的RPMI 1640培养基(Gibco,美国)中培养。
1.4 下调HepG2细胞miR-18B-5p表达 将HepG2细胞(5×105个)铺在6孔板上,在37 ℃、CO2体积分数为5%的条件下培养,直至细胞密度增至50%时加入附带嘌呤霉素抗性的病毒液(吉凯基因),病毒感染6 h换普通培养基培养,用嘌呤霉素浓度为0.6 μmol/L的全培养基筛选10 d,所得细胞为感染成功的细胞。通过qPCR检验其下调效果。
1.5 细胞实验CCK-8分析 将HepG2细胞接种于96孔板(5×105个/孔)中,在37 ℃和5%CO2中培养1~4 d,每孔加入20 μL CCK-8试剂,避光3 h,摇床振荡15 min。将酶标仪的波长设置为450 nm,检测CCK-8混合物的光密度(optical density,OD)值,以分析活性。重复测量3次,取平均值。
1.6 细胞实验Transwell分析 将对数生长期的HepG2细胞用胰蛋白酶消化后,再悬浮于无血清DMEM培养基中,将细胞密度调整为5×104个/mL,将600 μL含10% FBS的DMEM培养基加入转孔器下腔,将200 μL无血清细胞悬液用水合BD凝胶基膜加入上腔。培养24 h后,用磷酸缓冲盐溶液洗涤细胞2次,用4%多聚甲醛固定15 min,室温下风干。细胞用结晶紫染色10 min,磷酸缓冲盐溶液洗涤3次。用棉签轻轻擦拭上层的未迁移细胞,33%醋酸脱色,在570 nm处用酶标仪进行分析。重复测量3次,取平均值。
1.7 细胞实验定量实时RT-PCR 常规两步法提取HepG2细胞和患者血清中总RNA。miR-18B-5p扩增引物序列如下:miR-18B-5p的引物(引物序列为正向5′-GCGTAAGGTGCATCTAGTGCAG-3′反向5′-GTCGTATCCAGTGCAGGGTCCGAG-GTATTCGCACTGGATACGACCTAACT-3′)。U6,上游5′-CGCTTCGGCAGCACATATAC-3′,下游5′-CGAATTTGCGTGTCATCCTTG-3′。参考试剂盒说明书进行qPCR分析。PCR反应条件:95 ℃、5 min;95 ℃、30 s、60 ℃、30 s、72 ℃、30 s,共40个循环。miR-18B-5p mRNA的表达水平以2-△△Ct值表示。患者血清中miR-18B-5p表达水平根据相对表达水平的均值分为高miR-18B-5p表达组和低miR-18B-5p表达组。重复测量3次,取平均值。
1.8 统计学方法 应用SPSS 21.0统计软件分析数据。计量资料比较采用t检验、F检验、LSD-t检验,计数资料比较采用χ2检验或Fisher′s精确概率法,采用Kaplan-Meier法计算生存率,采用单因素和多因素Cox回归模型确定影响观察生存率的因素。P<0.05为差异有统计学意义。
2 结 果
2.1 高miR-18B-5p表达组和低miR-18B-5p表达组临床病理特征比较 高miR-18B-5p表达组和低miR-18B-5p表达组在性别、年龄、肝硬化患者占比和肝炎患者占比方面差异无统计学意义(P>0.05),见表1。
表1 miR-18B-5p与患者临床病理特征的关系
Table 1 The relationship between miR-18B-5p and clinical pathological characteristics of patients (例数)
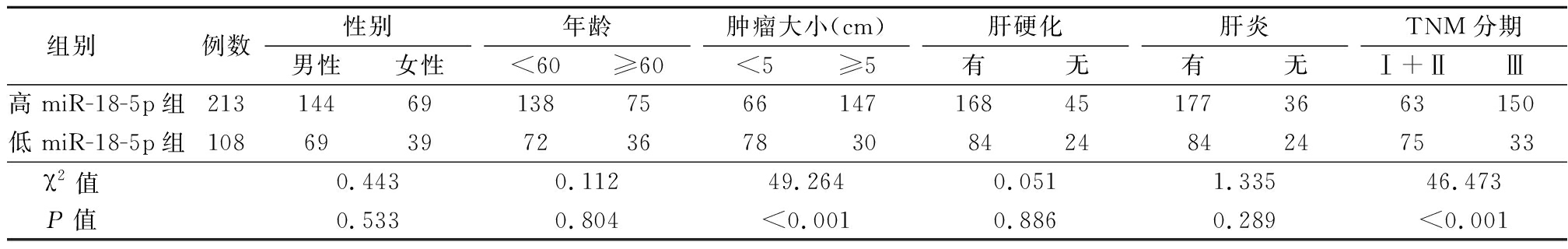
组别 例数性别男性女性年龄<60≥60肿瘤大小(cm)<5≥5肝硬化有无肝炎有无TNM分期Ⅰ+ⅡⅢ高miR-18-5p组213144691387566147168451773663150低miR-18-5p组108693972367830842484247533χ2值 0.4430.11249.2640.0511.33546.473P值 0.5330.804<0.0010.8860.289<0.001
2.2 高miR-18B-5p表达组和低miR-18B-5p表达组患者生存的比较 低miR-18B-5p表达组患者生存率高于高miR-18B-5p表达组,差异有统计学意义(P<0.05),见图1。
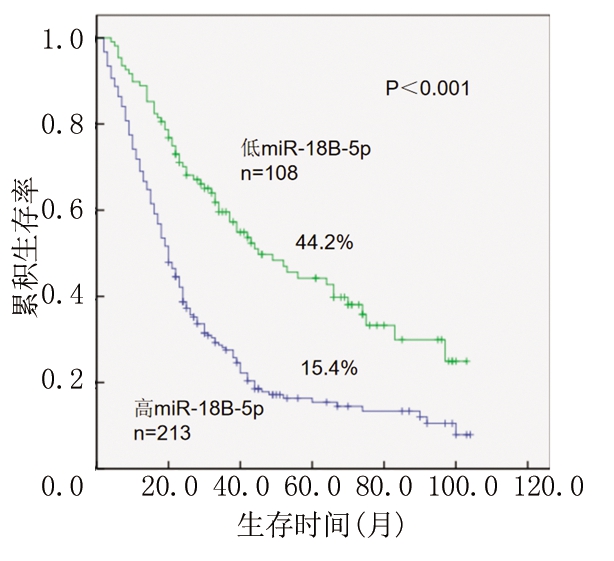
图1 miR-18B-5p表达的肝癌患者的总生存期
Figure 1 Overall survivals in hepatocellular carcinoma patients with miR-18B-5p expression
2.3 两组miR-18B-5p表达水平患者预后因素分析 单变量分析显示,年龄、肿瘤大小、TNM分期和miR-18B-5p(P<0.001)影响患者生存率,差异有统计学意义(P<0.05),见表2。
表2 肝癌患者生存的单因素分析
Table 2 Univariate analysis of survival in patients with hepatocellular carcinoma
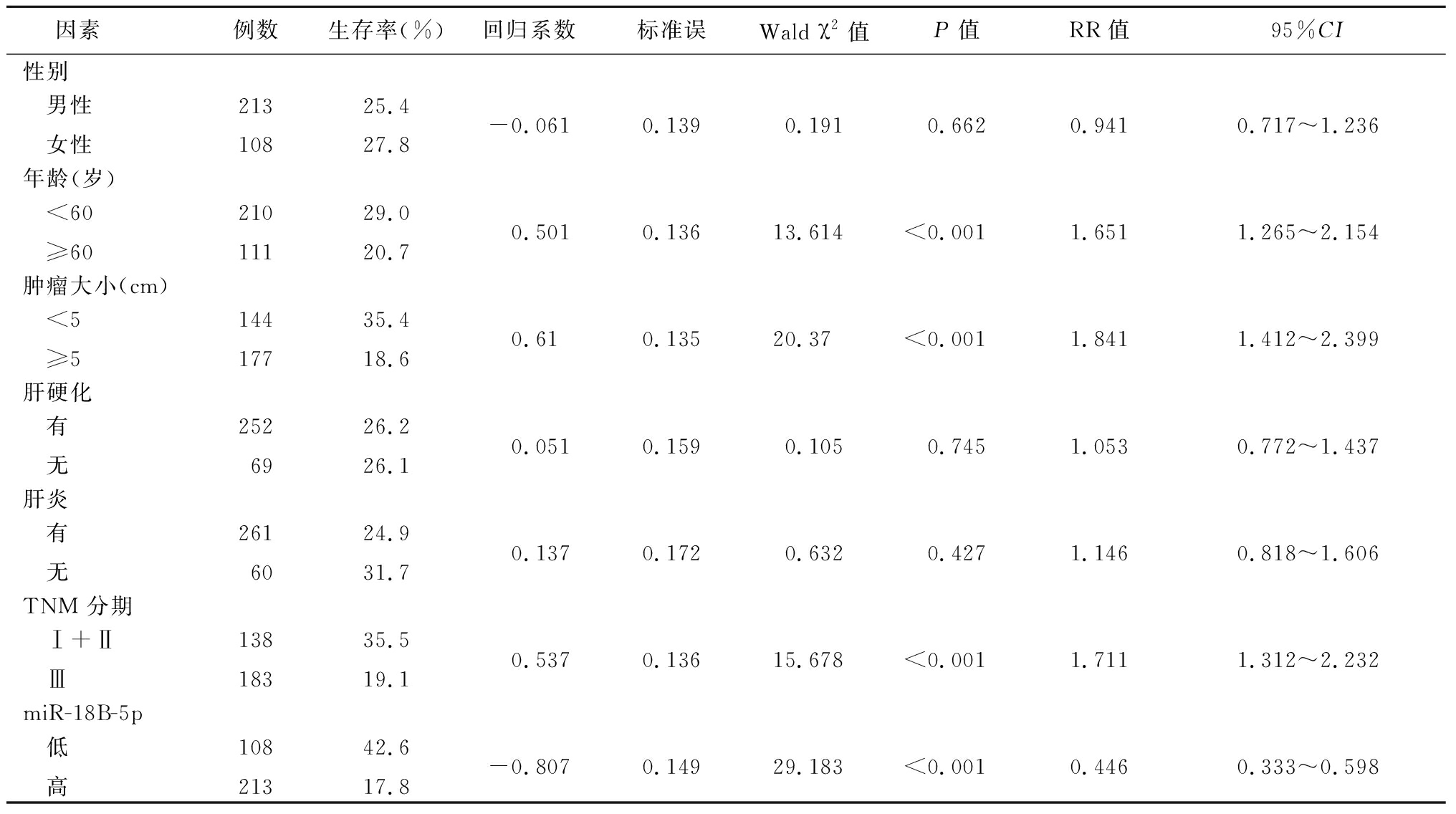
因素 例数生存率(%)回归系数标准误Wald χ2值P值RR值95%CI性别 男性 女性21310825.427.8-0.0610.1390.1910.6620.9410.717~1.236年龄(岁) <60 ≥6021011129.020.70.5010.13613.614<0.0011.6511.265~2.154肿瘤大小(cm) <5 ≥514417735.418.60.610.13520.37<0.0011.8411.412~2.399肝硬化 有 无2526926.226.10.0510.1590.1050.7451.0530.772~1.437肝炎 有 无2616024.931.70.1370.1720.6320.4271.1460.818~1.606TNM 分期 Ⅰ+Ⅱ Ⅲ13818335.519.10.5370.13615.678<0.0011.7111.312~2.232miR-18B-5p 低 高10821342.617.8-0.8070.14929.183<0.0010.4460.333~0.598
以年龄(<60岁=0,≥60岁=1)、肿瘤大小(<5 cm=0,≥5 cm=1);TNM 分期(Ⅰ+Ⅱ期=0,Ⅲ期=1);miR-18B-5p(低表达=0,高表达=1)为自变量,以患者生存时间为因变量,多因素分析结果显示,miR-18B-5p的表达水平(RR:2.064,95%CI:1.522~2.800)和年龄(RR:1.762,95%CI:1.347~2.305)是患者生存的影响因素,见表3。
表3 肝癌患者生存的多因素分析
Table 3 Multivariate analysis of survival in patients with hepatocellular carcinoma
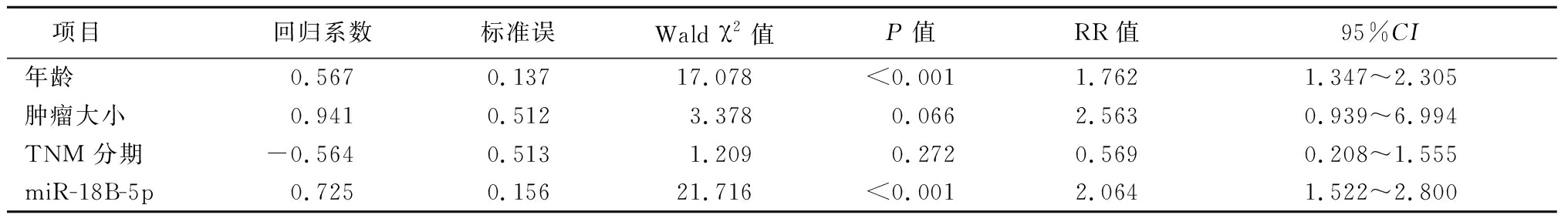
项目 回归系数标准误Wald χ2值P值RR值95%CI年龄0.5670.13717.078<0.0011.7621.347~2.305肿瘤大小0.9410.5123.3780.0662.5630.939~6.994TNM 分期-0.5640.5131.2090.2720.5690.208~1.555miR-18B-5p0.7250.15621.716<0.0012.0641.522~2.800
2.4 下调miR-18B-5p抑制肝癌细胞增殖、迁移和侵袭 HepG2细胞表达的miR-18B-5p水平高于Bel-7402组、MHCC97组和Hep3B组,差异有统计学意义(P<0.05),见表4。通过感染慢病毒的方法下调miR-18B-5p的表达,根据CCK-8实验结果,Si-miR-18B-5p细胞系的Si-miR-18B-5p表达和细胞增殖低于HepG2细胞系,差异有统计学意义(P<0.05),见表5,图2。用涂有或不涂基质凝胶的Transwell平板中的细胞检测细胞的迁移和侵袭水平,在Si-miR-18B-5p细胞系miR-18B-5p下调,细胞迁移和侵袭能力低于HepG2细胞系,差异有统计学意义(P<0.05),见表6。
表4 不同细胞系miR-18B-5p表达水平
Table 4 The expression level of miR-18B-5p in different cell lines ![]()
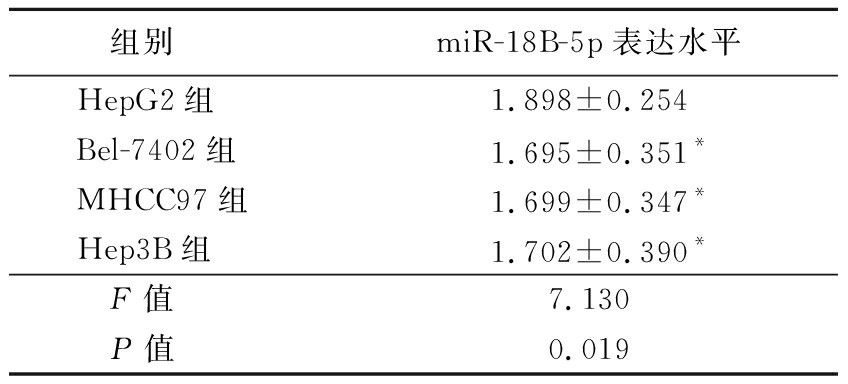
组别miR-18B-5p表达水平HepG2组1.898±0.254Bel-7402组1.695±0.351*MHCC97组1.699±0.347*Hep3B组1.702±0.390*F值7.130P值0.019
*P值<0.05与HepG2组比较(LSD-t检验)
表5 HepG2和Si-miR-18B-5p细胞中miR-18B-5p表达水平比较
Table 5 Comparison of miR-18B-5p expression levels in HepG2 and Si-miR-18B-5p cells ![]()

组别miR-18B-5p表达水平48 h细胞增殖水平HepG2组108.509±14.5008.052±0.179Si-miR-18B-5p组21.754±3.3624.263±0.459 t值47.12576.911 P值<0.001<0.001
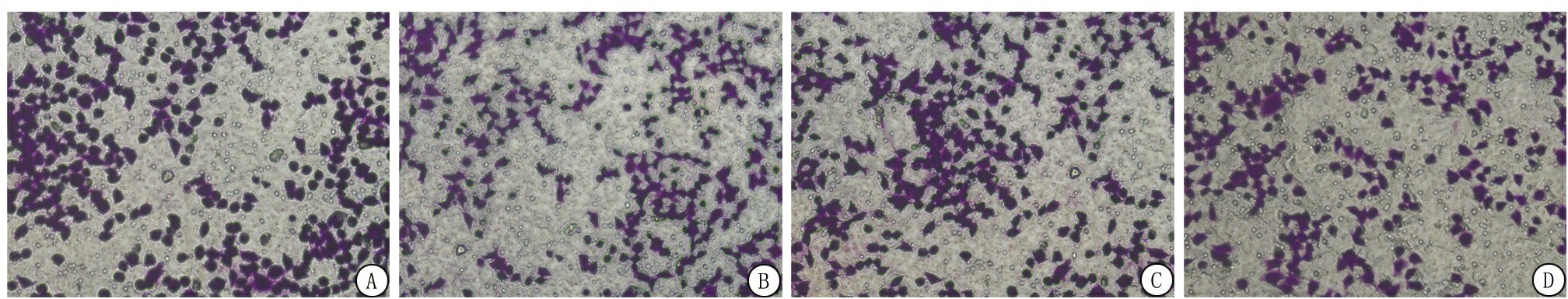
图2 HepG2和Si-miR-18B-5p细胞增殖水平比较(结晶紫染色× 400)
A.HepG2细胞迁移;B.Si-miR-18B-5p细胞迁移;C.HepG2细胞侵袭;D.Si-miR-18B-5p细胞侵袭
Figure 2 Comparison of proliferation levels in HepG2 and Si-miR-18B-5p cells(crystal violet staining× 400)
表6 HepG2和Si-miR-18B-5p细胞迁移能力和侵袭能力比较
Table 6 Comparison of migration ability and invasion ability of HepG2 and Si-miR-18B-5p cells ![]()
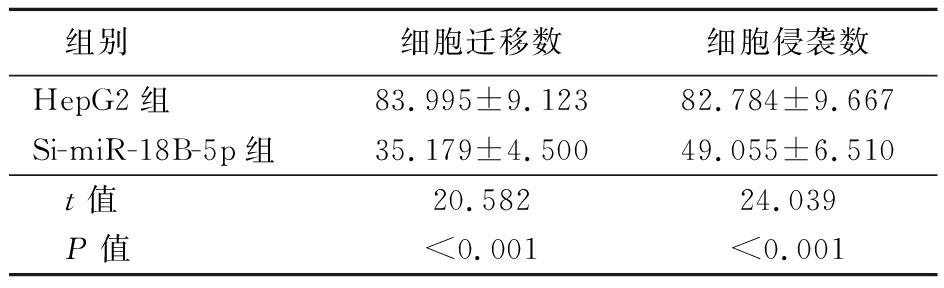
组别细胞迁移数细胞侵袭数HepG2组83.995±9.12382.784±9.667Si-miR-18B-5p组35.179±4.50049.055±6.510 t值 20.58224.039 P值<0.001<0.001
2.5 miR-18B-5p直接靶向BTG3调节BTG3表达 根据qRT-PCR,Si-miR-18B-5p细胞中BTG3的mRNA表达高于HepG2细胞系,差异有统计学意义(P<0.05),见表7。BTG3可能在miR-18B-5p的失调中起重要作用,构建了含有BTG3的荧光素酶报告基因,具有野生型(WT)或突变型(Mut)BTG3结合位点研究miR-18B-5p的靶点,结果显示,BTG3 WT相对荧光素活性低于BTG3 Mut,差异有统计学意义(P<0.05),见图3,表8。
表7 HepG2和Si-miR-18B-5p细胞BTG3 mRNA表达水平比较
Table 7 Comparison of BTG3 mRNA expression levels in HepG2 and Si-miR-18B-5p cells ![]()
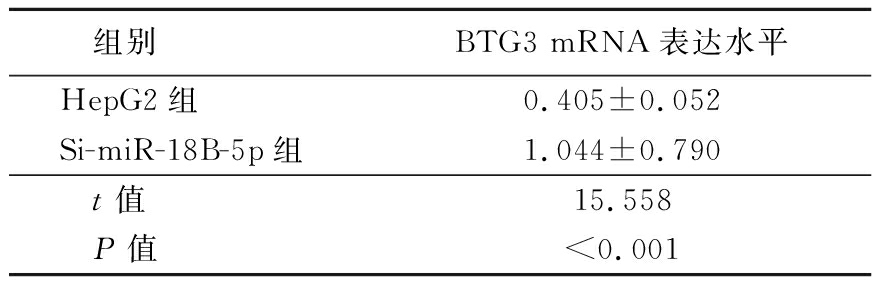
组别BTG3 mRNA表达水平HepG2组0.405±0.052Si-miR-18B-5p组1.044±0.790t值15.558P值<0.001
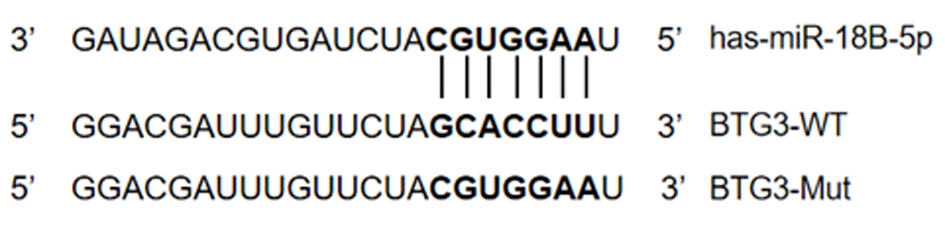
图3 BTG3结合位点碱基序列
Figure 3 Base sequence of BTG3 binding site
表8 不同组相对荧光素活性比较
Table 8 Comparison of relative fluorescein activity of different groups ![]()
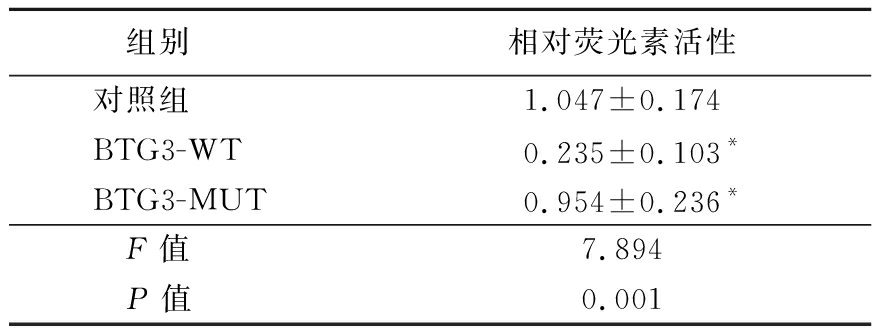
组别相对荧光素活性对照组1.047±0.174BTG3-WT0.235±0.103*BTG3-MUT0.954±0.236*F值7.894P值0.001
*P值<0.05与对照组比较(LSD-t检验)
3 讨 论
由于肝癌患者预后不佳,早期诊断和早期治疗有助于改善患者预后。既往文献显示miR-18B-5p在肝癌细胞中表达水平不同,有可能作为新的肝癌肿瘤标志物应用于临床,本研究旨在分析miR-18B-5p与肝癌的关系。
本研究结果显示,miR-18B-5p表达水平较低的肝细胞癌患者生存率较高,单因素分析结果表明血清中miR-18B-5p的表达水平与病变的大小、TNM分期及生存期相关,多因素分析显示miR-18B-5p高表达是肝癌患者预后不良的独立影响因素。Zhang等[14]研究认为患者血清miR-18B-5p表达水平可作为结直肠癌患者诊断的生物学指标。Cochetti等[15]报道血清中miR-18B-5p的表达水平可用于诊断前列腺增和前列腺癌。本研究结果与上述研究结论相似,表明血清中miR-18B-5p的表达水平可以应用于肝癌患者的临床诊断,并可以对患者的临床病理因素和预后进行预测。
本研究进一步通过体外实验探索miR-18B-5p在肝癌细胞中的作用机制,通过慢病毒感染的方法下调HepG2中miR-18B-5p的表达,抑制了肝癌细胞的增殖、迁移和侵袭。与此研究结果不同,Xue等[16]报道miR-18B-5p可降低卵巢癌细胞的增殖和转移,抑制癌细胞的生长发育。Xue等[17]研究表明miR-18b-5p可通过VMA21轴抑制肺腺癌的增殖。另有研究认为miR-18b-5p能增强乳腺癌细胞的迁移和侵袭能力,其作用机制是抑制细胞分裂因子4的表达[18]。本研究结果证明miR-18B-5p在肝癌细胞中可以促进其增殖、迁移和侵袭。
本研究检测BTG3在HepG2和Si-miR-18B-5p细胞中的表达,结果显示,BTG3在Si-miR-18B-5p细胞中的表达水平是HepG2细胞的2.578倍。下调miR-18B-5p表达后,显示后者能促进BTG3的表达。免疫荧光酶报告基因实验结果显示,MiR-18b-5p通过结合靶基因BTG3的mRNA上的3′-UTR抑制BTG3,从而促进肝癌的增殖和转移。BTG3是抗增殖蛋白家族的B细胞转运体基因/转运体的成员[19],是细胞周期和细胞增殖重要的负向调节剂[20-22]。BTG3与多种肿瘤的进展和转移有关,可通过与E2F1结合而负调节细胞生长[21]。研究显示,BTG3可以通过调节AKT /GSK3β/β-Catenin通路阻止肿瘤的进展[23]。因此,miR-18b-5p通过靶向BTG3促进肝细胞癌的增殖、侵袭和转移可作为今后靶向治疗的新思路。
综上所述,miR-18b-5p的临床意义在于其可用于肝癌的早期诊断和预测预后,尤其是基于血清miR-18b-5p的表达水平。血清miRNA检测具有操作简单、标本易取、价格低廉等优点,更适合临床工作。
[1] Meng PP,Zhang YF,Zhang WL,et al. Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells[J]. Sci Rep,2021,11(1):40.
[2] Hsu WF,Chuang PH,Chen CK,et al. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience[J]. Am J Cancer Res,2020,10(12):4547-4560.
[3] Chen Q,Zhou XW,Zhang AJ,et al. ACTN1 supports tumor growth by inhibiting Hippo signaling in hepatocellular carcinoma[J]. J Exp Clin Cancer Res,2021,40(1):23.
[4] Zhang HB,Yi JK,Yoon D,et al. Imatinib and GNF-5 exhibit an inhibitory effect on growth of hepatocellar carcinoma cells by downregulating s-phase kinase-associated protein 2[J]. J Cancer Prev,2020,25(4):252-257.
[5] Bray F,Ferlay J,Soerjomataram I,et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2018,68(6):394-424.
[6] Zhou TH,Su JZ,Qin R,et al. Prognostic and predictive value of a 15 transcription factors(TFs) Panel for hepatocellular carcinoma[J]. Cancer Manag Res,2020,12: 12349-12361.
[7] Ponziani FR,Bhoori S,Castelli C,et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease[J]. Hepatology,2019,69(1):107-120.
[8] Deng P,Wu YN. Knockdown of miR-106a suppresses migration and invasion and enhances radiosensitivity of hepatocellular carcinoma cells by upregulating FBXW7[J]. Int J Clin Exp Pathol,2019,12(4):1184-1193.
[9] Liu C,Shang ZH,Ma Y,et al. HOTAIR/miR-214-3p/FLOT1 axis plays an essential role in the proliferation,migration,and invasion of hepatocellular carcinoma[J]. Int J Clin Exp Pathol,2019,12(1):50-63.
[10] Cao LQ,Yang XW,Chen YB,et al. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth[J]. Mol Cancer,2020,19(1):59.
[11] Mo YC,He LG,Lai Z,et al. Gold nano-particles(AuNPs) carrying miR-326 targets PDK1/AKT/c-myc axis in hepatocellular carcinoma[J]. Artif Cells Nanomed Biotechnol,2019,47(1):2830-2837.
[12] Yu M,Xue HZ,Wang YD,et al. miR-345 inhibits tumor metastasis and EMT by targeting IRF1-mediated mTOR/STAT3/AKT pathway in hepatocellular carcinoma[J]. Int J Oncol,2017,50(3):975-983.
[13] Chen W,Huang L,Liang JH,et al. Long noncoding RNA small nucleolar RNA host gene 15 deteriorates liver cancer via microRNA-18b-5p/LIM-only 4 axis[J]. IUBMB Life,2021,73(2):349-361.
[14] Zhang H,Zhu MX,Shan X,et al. A panel of seven-miRNA signature in plasma as potential biomarker for colorectal cancer diagnosis[J]. Gene,2019,687:246-254.
[15] Cochetti G,Poli G,Guelfi G,et al.Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: evaluation of potential diagnostic and prognostic role[J]. Onco Targets Ther,2016,9:7545-7553.
[16] Xue F,Xu YH,Shen CC,et al.Non-coding RNA LOXL1-AS1 exhibits oncogenic activity in ovarian cancer via regulation of miR-18b-5p/VMA21 axis[J]. Biomed Pharmacother,2020,125:109568.
[17] Xue M,Tao WM,Yu SY,et al. lncRNA ZFPM2-AS1 promotes proliferation via miR-18b-5p/VMA21 axis in lung adenocarcinoma[J]. J Cell Biochem,2020,121(1):313-321.
[18] Wang YY,Yan L,Yang S,et al. Long noncoding RNA AC073284.4 suppresses epithelial-mesenchymal transition by sponging miR-18b-5p in paclitaxel-resistant breast cancer cells[J]. J Cell Physiol,2019,234(12):23202-23215.
[19] Cheng YC,Chiang HY,Cheng SJ,et al. Loss of the tumor suppressor BTG3 drives a pro-angiogenic tumor microenvironment through HIF-1 activation[J]. Cell Death Dis,2020,11(12):1046.
[20] Peng LJ,Li SB,Li YC,et al. Regulation of BTG3 by microRNA-20b-5p in non-small cell lung cancer[J]. Oncol Lett, 2019,18(1):137-144.
[21] Lv C,Wang HL,Tong YX,et al. The function of BTG3 in colorectal cancer cells and its possible signaling pathway[J]. J Cancer Res Clin Oncol,2018,144(2):295-308.
[22] Liu LQ,Liu SC,Duan QX,et al. MicroRNA-142-5p promotes cell growth and migration in renal cell carcinoma by targeting BTG3[J]. Am J Transl Res,2017,9(5):2394-2402.
[23] An Q,Zhou Y,Han C,et al. BTG3 Overexpression suppresses the proliferation and invasion in epithelial ovarian cancer cell by regulating AKT/GSK3β/β-Catenin signaling[J]. Reprod Sci,2017,24(10):1462-1468.