褥疮即压力性损伤,在世界范围内被列为严重伤害患者的五大因素之一,亦是花费最高的一种并发症,给患者、家庭、社会带来沉重的经济和医疗负担[1-2]。脑梗死患者由于年龄较大、病情危重、长期卧床、合并糖尿病等,导致组织受压、缺血、坏死,是褥疮及高分度褥疮的高发人群,深入了解脑梗死患者继发Ⅲ、Ⅳ度褥疮感染的相关机制,早期预测创面愈合情况,对指导临床决策、干预等具有重要意义[3-4]。免疫黏附促进因子(forming enhancement rosetterate,FEER)、红细胞C3b受体花环率(erythrocyte C3b receptor wreath rate,RBC-C3bR)均为红细胞免疫指标,在伤口感染的发生及机体免疫应答中起到重要作用[5]。中性粒细胞主要功能是负责人体的免疫功能,能调节血管通透性、疼痛、血液凝固、炎症反应等,在褥疮患者皮肤及皮下组织中均可检测到中性粒细胞浸润[6-7]。白细胞介素6(interleukin-6,IL-6)是一种促炎因子,可加重褥疮创面炎症反应,减缓创面愈合速度[8]。现阶段关于FEER、中性粒细胞百分比(neutrophil%,NEU%)、IL-6、RBC-C3bR在脑梗死继发Ⅲ、Ⅳ度褥疮感染患者中表达及与预测创面愈合价值的研究鲜见,本研究对此进行探讨,旨在为临床诊治疾病提供参考,报告如下。
1 资料与方法
1.1 一般资料 选取2018年4月—2020年2月北京市房山区良乡医院收治的43例脑梗死继发Ⅲ度褥疮感染者(Ⅲ度组)、43例脑梗死继发Ⅳ度褥疮感染者(Ⅳ度组)及43例正常人群(对照组)作为研究对象。纳入标准:①病例均符合压力性损伤诊断标准[9];②褥疮分度Ⅲ、Ⅳ度;继发于脑梗死后;③入组前无相关治疗史。排除标准:①合并肺部感染等其他感染类疾病者;②自身免疫疾病者;③癌症患者;④血液系统疾病者;⑤出血倾向或活动性出血者。
本研究获医院伦理委员会审核批准,患者及家属知情同意并签署知情同意书。
1.2 方法
1.2.1 资料收集 收集入组者性别、年龄、体重指数、脑梗死病程、饮酒史、吸烟史、合并疾病、神经缺损程度资料,其中神经缺损程度根据发病时美国国立卫生研究院卒中量表(National Institute of Health Stroke Scale,NIHSS)评估,评分5~15分为中度,16~42分为重度,采用Epi Data 3.02双人双录,保证数据录入的准确性。
1.2.2 褥疮分度标准[9] Ⅲ度:损伤到达真皮层,有明显渗出和感染,出现组织坏死,无明显疼痛;Ⅳ度:损伤达肌腱或骨质,明显渗出和感染,大量组织坏死,伴或不伴疼痛。
1.2.3 治疗方法 均采用负压封闭引流联合敏感抗菌药物进行治疗,先清除坏死组织,彻底消毒创面及周围组织,再给予负压封闭引流,有潜行与窦道伤口用爱康肤银敷料填塞,外贴棉垫覆盖保湿材料,妥善固定,更换敷料的时机是外层敷料被渗出液湿透2/3时。治疗10 d、20 d时分别对创面愈合情况进行评估,待创面情况改善后实施Ⅱ期修复手术。
1.2.4 创面愈合标准[9] 创面无渗液和坏死组织,无水肿,完全愈合,肉芽组织生长呈新鲜、粉红颗粒状,有植皮和皮瓣转移条件。
1.2.5 褥疮愈合计分量表(pressure ulcer scale for healing,PUSH)[10] PUSH量表包含创面面积、24 h渗液、组织形态,最高分17分,分值越高,愈合状态越差。
1.2.6 各指标检测 分别于治疗前、治疗10 d、治疗20 d采集患者肘部静脉血5 mL,采用全自动生化分析仪(美国MD公司魅力2000型)检测NEU%;并取标本3 000 r/min离心5 min,留取血清和下层红细胞,采用酶联免疫吸附法检测血清IL-6,试剂盒购于美国罗氏公司;采用流式细胞仪(美国Coulter EPICSXL型)检测下层红细胞FEER、RBC-C3bR水平。
1.3 观察指标 ①比较各组一般资料、FEER、NEU%、IL-6、RBC-C3bR水平。②分析FEER、NEU%、IL-6、RBC-C3bR与褥疮分度的关系。③比较创面愈合与未愈合者FEER、NEU%、IL-6、RBC-C3bR水平。④比较创面愈合与未愈合者PUSH评分,分析FEER、NEU%、IL-6、RBC-C3bR与PUSH评分的相关性。⑤分析FEER、NEU%、IL-6、RBC-C3bR预测创面愈合的价值。
1.4 统计学方法 应用SPSS 22.0统计学软件处理数据。计量资料两组间比较采用t检验,多组间比较采用单因素方差分析,两两比较采用LSD-t检验,不同时间点、组别交互作用下FEER、NEU%、IL-6、RBC-C3bR采用重复测量方差分析法检验,计数资料比较采用χ2检验,Spearman分析FEER、NEU%、IL-6、RBC-C3bR与褥疮分度的关系,采用Pearson分析FEER、NEU%、IL-6、RBC-C3bR与PUSH评分的相关性,采用受试者工作特征曲线(receiver operating characteristic,ROC)及ROC下面积(area under the curve,AUC)分析FEER、NEU%、IL-6、RBC-C3bR预测创面愈合的价值。P<0.05为差异有统计学意义。
2 结 果
2.1 各组一般资料、FEER、NEU%、IL-6、RBC-C3bR比较 各组年龄41~84岁,组间性别、年龄、体重指数、脑梗死病程、饮酒史、吸烟史、合并疾病、神经缺损程度比较差异无统计学意义(P>0.05);FEER、RBC-C3bR:Ⅳ度组<Ⅲ度组<对照组,NEU%、IL-6:Ⅳ度组>Ⅲ度组>对照组,组间比较差异有统计学意义(P<0.05)。见表1。
表1 各组一般资料、FEER、NEU%、IL-6、RBC-C3bR比较
Table 1 Comparison of general data, FEER, NEU%, IL-6, and RBC-C3bR of each group (n=43)
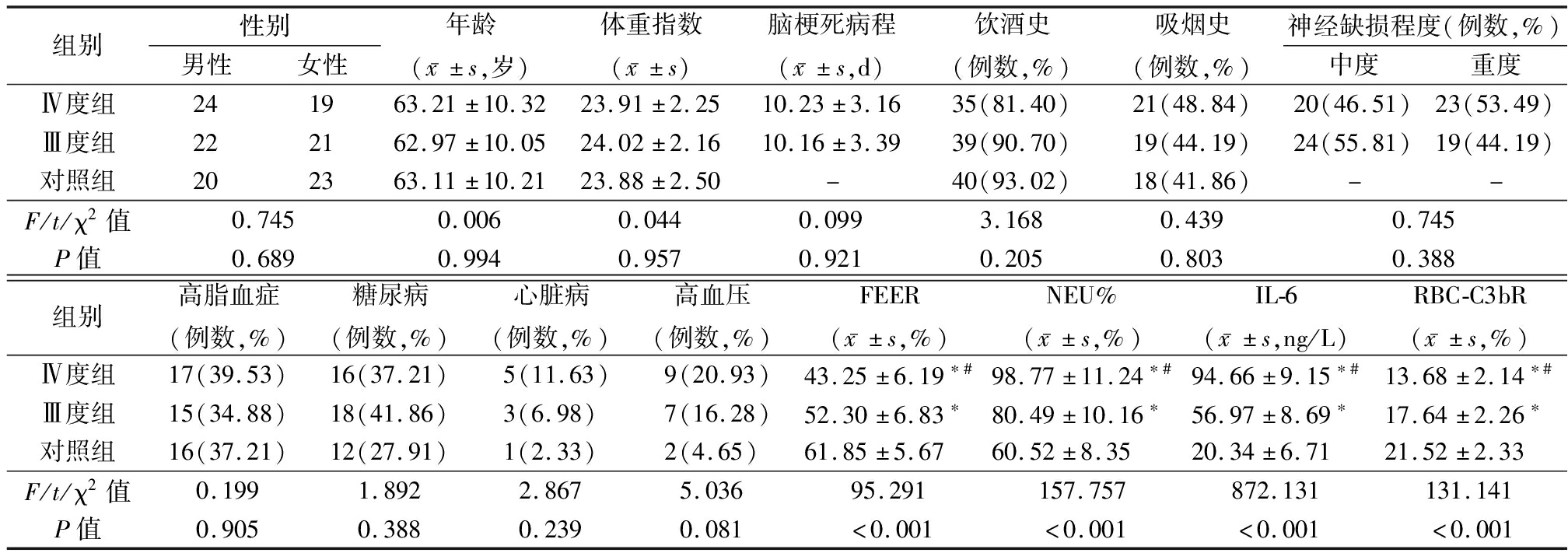
组别性别男性女性年龄(x-±s,岁)体重指数(x-±s)脑梗死病程(x-±s,d)饮酒史(例数,%)吸烟史(例数,%)神经缺损程度(例数,%)中度重度Ⅳ度组241963.21±10.3223.91±2.2510.23±3.1635(81.40)21(48.84)20(46.51)23(53.49)Ⅲ度组222162.97±10.0524.02±2.1610.16±3.3939(90.70)19(44.19)24(55.81)19(44.19)对照组202363.11±10.2123.88±2.50-40(93.02)18(41.86)--F/t/χ2值0.7450.0060.0440.0993.1680.4390.745P值0.6890.9940.9570.9210.2050.8030.388组别高脂血症(例数,%)糖尿病(例数,%)心脏病(例数,%)高血压(例数,%)FEER(x-±s,%)NEU%(x-±s,%)IL-6(x-±s,ng/L)RBC-C3bR(x-±s,%)Ⅳ度组17(39.53)16(37.21)5(11.63)9(20.93)43.25±6.19∗#98.77±11.24∗#94.66±9.15∗#13.68±2.14∗#Ⅲ度组15(34.88)18(41.86)3(6.98)7(16.28)52.30±6.83∗80.49±10.16∗56.97±8.69∗17.64±2.26∗对照组16(37.21)12(27.91)1(2.33)2(4.65)61.85±5.6760.52±8.3520.34±6.7121.52±2.33F/t/χ2值0.1991.8922.8675.03695.291157.757872.131131.141P值0.9050.3880.2390.081<0.001<0.001<0.001<0.001
“-”表示无此项资料 *P值<0.05与对照组比较 #P值<0.05与Ⅲ度组比较(LSD-t检验)
2.2 FEER、NEU%、IL-6、RBC-C3bR与褥疮分度的关系 FEER、RBC-C3bR与褥疮分度呈负相关,NEU%、IL-6与褥疮分度呈正相关(P<0.05)。见表2。
表2 FEER、NEU%、IL-6、RBC-C3bR与褥疮分度的关系
Table 2 Relationship between FEER, NEU%,IL-6,RBC-C3bR and the degree of bedsore
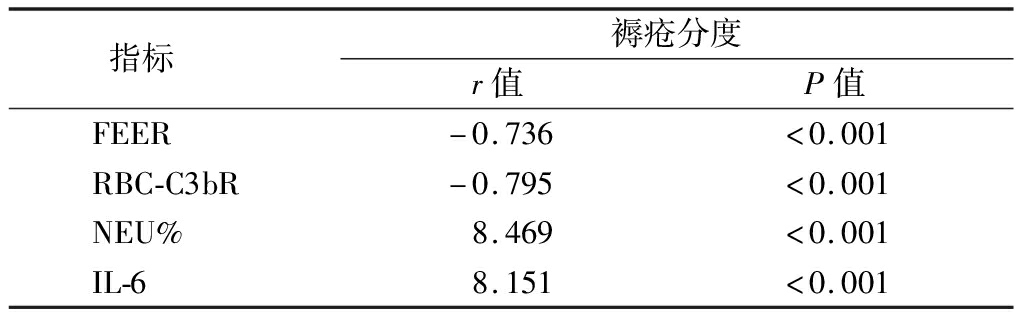
指标 褥疮分度r值P值FEER-0.736<0.001RBC-C3bR-0.795<0.001NEU%8.469<0.001IL-68.151<0.001
2.3 创面愈合与未愈合者FEER、NEU%、IL-6、RBC-C3bR比较 不同时间点、组间、组间·不同时间点FEER、NEU%、IL-6、RBC-C3bR比较,差异均有统计学意义(P<0.05);治疗10 d创面愈合者FEER、RBC-C3bR呈升高趋势,NEU%、IL-6呈降低趋势,且治疗20 d各指标与治疗10 d比较,差异有统计学意义(P<0.05);创面未愈合者治疗10 d FEER、RBC-C3bR高于治疗前,NEU%、IL-6低于治疗前(P<0.05);创面愈合者治疗10 d、20 d FEER、RBC-C3bR高于未愈合者,NEU%、IL-6低于未愈合者(P<0.05)。见表3。
表3 创面愈合与未愈合者FEER、NEU%、IL-6、RBC-C3bR比较
Table 3 Comparison of FEER, NEU%, IL-6, and RBC-C3bR between wound healed and unhealed patients![]()
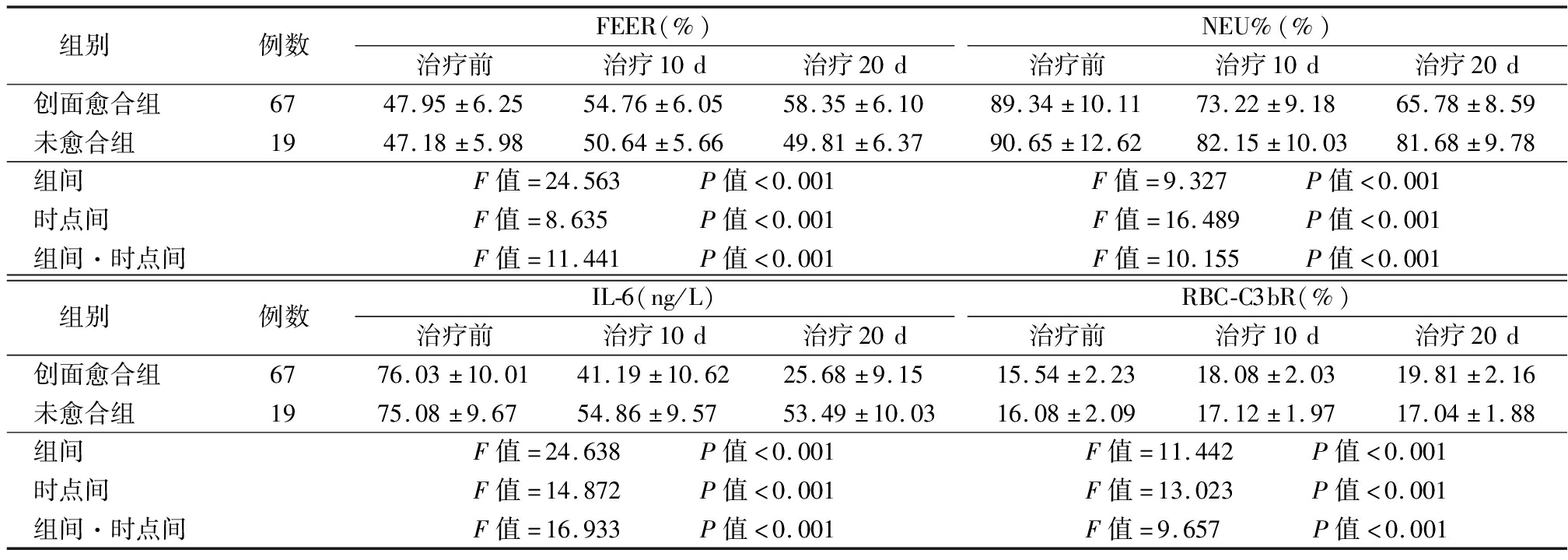
组别 例数FEER(%)治疗前治疗10d治疗20dNEU%(%)治疗前治疗10d治疗20d创面愈合组 6747.95±6.2554.76±6.0558.35±6.1089.34±10.1173.22±9.1865.78±8.59未愈合组 1947.18±5.9850.64±5.6649.81±6.3790.65±12.6282.15±10.0381.68±9.78组间 F值=24.563 P值<0.001F值=9.327 P值<0.001时点间 F值=8.635 P值<0.001F值=16.489 P值<0.001组间·时点间F值=11.441 P值<0.001F值=10.155 P值<0.001组别 例数IL-6(ng/L)治疗前治疗10d治疗20dRBC-C3bR(%)治疗前治疗10d治疗20d创面愈合组 6776.03±10.0141.19±10.6225.68±9.1515.54±2.2318.08±2.0319.81±2.16未愈合组 1975.08±9.6754.86±9.5753.49±10.0316.08±2.0917.12±1.9717.04±1.88组间 F值=24.638 P值<0.001F值=11.442 P值<0.001时点间 F值=14.872 P值<0.001F值=13.023 P值<0.001组间·时点间F值=16.933 P值<0.001F值=9.657 P值<0.001
2.4 FEER、NEU%、IL-6、RBC-C3bR与PUSH评分的相关性 创面愈合者治疗10 d与20 d PUSH评分低于未愈合者(P<0.05)。治疗10 d与20 d FEER(r=-0.897、-0.629,P<0.001)、RBC-C3bR(r=-0.708、-0.513,P<0.001)与PUSH评分呈负相关,NEU%(r=0.826、0.701,P<0.001)、IL-6(r=0.850、0.766,P<0.001)与PUSH评分呈正相关(P<0.05),且治疗10 d的相关性强于治疗20 d,见表4。
表4 创面愈合与未愈合者PUSH评分比较
Table 4 Comparison of PUSH scores between wound healed and unhealed patients ![]() 分)
分)
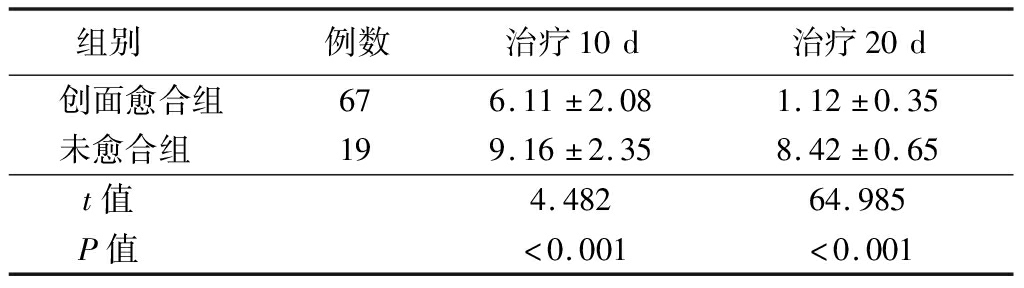
组别 例数治疗10d治疗20d创面愈合组676.11±2.081.12±0.35未愈合组 199.16±2.358.42±0.65t值 4.48264.985P值 <0.001<0.001
2.5 FEER、NEU%、IL-6、RBC-C3bR预测创面愈合的价值 由于治疗10 d FEER、NEU%、IL-6、RBC-C3bR与PUSH评分的相关性较强,故采用治疗10 d各指标水平预测创面愈合,通过ROC分析可知,单一指标中,IL-6预测创面愈合的AUC最大(0.823),各指标联合预测创面愈合的AUC为0.896,大于任一单一指标,见图1、表5。
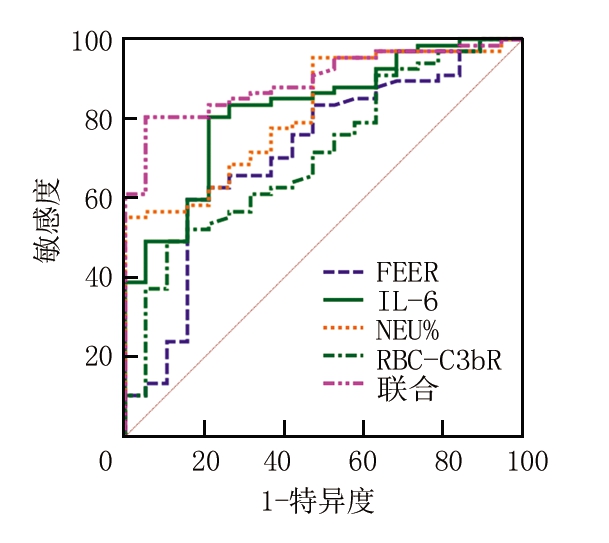
图1 FEER、NEU%、IL-6、RBC-C3bR预测创面愈合的价值
Figure 1 Value of FEER,NEU%,IL-6,and RBC-C3bR in predicting wound healing
表5 ROC分析结果
Table 5 ROC analysis results
指标 AUC95%CIP值cut-off值敏感度(%)特异度(%)FEER0.7140.576~0.853<0.001>53.28%59.7084.21NEU%0.8190.721~0.916<0.001≤71.65%55.2299.87IL-60.8230.722~0.924<0.001≤49.02ng/L80.6078.95RBC-C3bR0.7090.581~0.838<0.001>18.87%49.2589.47联合0.8960.829~0.963<0.001-80.6094.74
3 讨 论
以往观点认为,红细胞主要功能是运输氧气和二氧化碳,但随着相关研究推进,人们发现红细胞表面存在天然免疫分子,可清除循环免疫复合物、识别黏附、杀伤抗原,在免疫调节中占有很重要的地位[11-12]。FEER、RBC-C3bR均为红细胞免疫因子,在急性喉炎等炎症类疾病及轮状病毒感染等疾病中表达显著降低,并与疾病严重程度有关[13-14]。但在脑梗死后褥疮感染领域的报道鲜见,本研究创新性探讨发现,FEER、RBC-C3bR在脑梗死继发Ⅲ、Ⅳ度褥疮感染患者中表达明显降低,与褥疮分度呈负相关,参与了脑梗死继发Ⅲ、Ⅳ度褥疮感染发生及病情的恶化。脑梗死继发Ⅲ、Ⅳ度褥疮感染患者局部皮肤组织糜烂、坏死、出血,造成红细胞数量减少,引起FEER、RBC-C3bR水平降低,使免疫复合物清除受阻,又抑制了红细胞天然免疫能力,为褥疮感染的发生创造了有利条件,形成恶性循环,故亦与褥疮分度有关。FEER、RBC-C3bR水平增加,能增强红细胞免疫功能,有助于抵抗褥疮感染,从而加快创面愈合。后续的ROC分析显示,FEER、RBC-C3bR预测创面愈合的AUC分别为0.714、0.709,呈现出一定预测价值,能为临床早期预测创面愈合提供参考,从而指导临床干预。
中性粒细胞是人体内数量最多的白细胞类型,来源于骨髓,具有杀菌、吞噬、趋化等作用,当机体存在炎症时,其被趋化性物质吸引至炎症部位,在血流不畅、肿胀、缺氧情况下,仍可生存,在非特异性细胞免疫系统中扮演重要角色[15-16]。本研究结果显示,Ⅲ度组、对照组、Ⅳ度组NEU%依次升高,NEU%与褥疮分度、PUSH评分呈正相关,表明NEU%参与了脑梗死继发Ⅲ、Ⅳ度褥疮感染,并与病情程度、创面愈合有关。郭声敏等[17]报道,降低NEU%可缩短褥疮患者创面愈合时间,改善感染程度,本研究观点与之相似。褥疮患者皮肤受损,存在局部炎症反应,中性粒细胞被激活,故较正常人群明显升高,NEU%被趋化至受损部位,在酶作用下,生成前列腺素、血栓素,使血管通透性增加,引起疼痛、炎症,从而影响创面愈合[18-20]。因此对褥疮患者,可采取一定措施降低NEU%,以改善患者病情程度,加快创面愈合,同时治疗褥疮时,动态监测NEU%有助于疗效的评估。由于治疗10 d NEU%与PUSH评分的相关性较强,故采用治疗10 d NEU%水平预测创面愈合,发现NEU%预测创面愈合的AUC为0.819,呈现出较高的预测价值。
IL-6是多种感染类疾病中研究的热点指标之一,被证实具有加重、放大炎症反应的作用[21-22]。本研究结果显示,Ⅲ度组、对照组、Ⅳ度组IL-6依次升高,IL-6与褥疮分度呈正相关,提示IL-6参与了脑梗死继发Ⅲ、Ⅳ度褥疮感染,并与褥疮分度有关。兰园淞等[23]报道,IL-6在化疗大鼠褥疮中表达升高,并与皮肤肌肉损伤程度分布呈正相关,本研究结论与之相似。且本研究还发现,IL-6与创面愈合有关。采用相关药物降低Ⅲ、Ⅳ期褥疮患者IL-6表达,可缩小褥疮总面积,促进创面愈合[24]。脑梗死继发Ⅲ、Ⅳ度褥疮感染患者,褥疮部位组织坏死严重,伴有明显红肿热,可刺激IL-6的产生,并进一步加重脓肿状态,采用负压引流等治疗后,IL-6等炎性因子显著减少,有效抑制了创面炎症反应,为创面愈合提供了有利条件[25]。IL-6这一动态变化特点提示,对IL-6进行监测,能及时评估褥疮治疗效果,呈持续降低趋势常提示创面趋于愈合。同时采用具有抗炎作用敷料或药物等,抑制IL-6表达,有助于患者病情恢复。ROC分析显示,单一指标中,IL-6预测创面愈合的AUC最大,能为临床预测创面愈合提供可靠参考。
综上所述,脑梗死继发Ⅲ、Ⅳ度褥疮感染患者治疗前FEER、RBC-C3bR表达显著降低,NEU%、IL-6表达显著升高,治疗后FEER、RBC-C3bR升高及NEU%、IL-6降低可预示创面趋于愈合,检测治疗后10 d各指标水平有望成为预测创面愈合的生物标志物。
[1] Mervis JS,Phillips TJ.Pressure ulcers:pathophysiology,epidemiology,risk factors,and presentation[J].J Am Acad Dermatol,2019,81(4):881-890.
[2] Sumarno AS.Pressure ulcers:the core,care and cure approach[J].Br J Community Nurs,2019,24(Suppl 12):S38-S42.
[3] 黄凤英,秦秀英,宁炳雯.集束化护理干预在脑卒中患者预防压疮发生的临床效果评价[J].护理实践与研究,2021,18(1):51-53.
[4] 林秀娇,万琼红,胡荣.重症脑卒中患者压力性损伤发生特征及影响因素分析[J].护理学杂志,2020,35(1):41-44.
[5] 刘宁,支慧,单单单,等.红细胞免疫与脊柱术后患者伤口感染相关性研究[J].中华医院感染学杂志,2019,29(16):2486-2490,2494.
[6] Yao J,Zheng J,Cai J,et al.Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response[J].FASEB J,2019,33(2):1695-1710.
[7] 镇艳,易伟民,王华军,等.缺血处理对大鼠缺血再灌注性压疮中性粒细胞浸润度的影响[J].岭南急诊医学杂志,2018,23(1):17-19,23.
[8] 戢艳琼,郭俐宏,李梓香,等.瑰及乳膏对大鼠III期压疮模型纤维结合蛋白及碱性成纤维细胞生长因子的影响[J].中国比较医学杂志,2018,28(8):90-94.
[9] 杨龙飞,宋冰,倪翠萍,等.2019版《压力性损伤的预防和治疗:临床实践指南》更新解读[J].中国护理管理,2020,20(12):1849-1854.
[10] 周如女,张伟英,唐月红,等.压疮愈合计分量表在老年住院患者2期及以上压力性损伤中的应用研究[J].解放军护理杂志,2019,36(9):53-56.
[11] 黄振华,邓向亮,张凯敏,等.枸杞多糖对免疫抑制小鼠红细胞免疫功能的影响[J].中国免疫学杂志,2018,34(2):214-217.
[12] 卫芳征.外周血T细胞亚群和红细胞免疫功能与重症肺结核患者不良预后的相关性[J].临床肺科杂志,2018,23(9):1561-1565.
[13] 殷佟,袁博,黄雅玲,等.红细胞免疫及血清炎性因子水平与轮状病毒肠炎患儿脱水程度的关系[J].中国医药导报,2019,16(6):86-89.
[14] 隋丽丽,孟娟,孙平.急性喉炎患儿T淋巴细胞亚群差异性分析[J].中国妇幼健康研究,2018,29(3):284-287.
[15] Nakamura K,Kageyama S,Kupiec-Weglinski JW.The evolving role of neutrophils in liver transplant ischemia-reperfusion injury[J].Curr Transplant Rep,2019,6(1):78-89.
[16] Uderhardt S,Martins AJ,Tsang JS,et al.Resident macrophages cloak tissue microlesions to prevent neutriophil-driven inflammatory damage[J].Cell,2019,177(3):541-555.
[17] 郭声敏,张弛,黄厚强,等.肌骨超声引导下清创术对伴潜行Ⅲ~Ⅳ期压力性损伤患者的临床应用[J].国际护理学杂志,2019,38(24):4127-4131.
[18] Van Damme N,Van Hecke A,Remue E,et al.Physiological processes of inflammation and edema initiated by sustained mechanical loading in subcutaneous tissues:a scoping review[J].Wound Repair Regen,2020,28(2):242-265.
[19] 商安全,孙祖俊,李冬.中性粒细胞胞外陷阱及其在炎性损伤中的作用[J].现代免疫学,2020,40(5):419-423.
[20] 龙玉兰.康惠尔透明贴预防关节镜下前交叉韧带重建术患者压疮的疗效分析[J].中国医药科学,2019,9(1):91-94.
[21] 安辉,应秀东,方晶,等.黄芩苷对大鼠压疮模型的干预作用及机制[J].锦州医科大学学报,2020,41(4):22-29.
[22] 蒋舒玉,马加威.ICU危重症患者压力性损伤的影响因素分析[J].中华灾害救援医学,2020,8(3):178-180.
[23] 兰园淞,廖海涛,韦义萍,等.hs-CRP、TNF-α和 IL-6在化疗大鼠压疮形成中的表达和相关性研究[J].护理研究,2018,32(12):1862-1869.
[24] 朱爱萍,刘虹梅.托里透脓汤与冰片加诺氟沙星联合治疗Ⅲ期、Ⅳ期压疮的临床效果及对其血清TNF-α、IL-6水平的影响[J].四川中医,2018,36(4):75-78.
[25] Taradaj J,Shay B,Dymarek R,et al.Effect of laser therapy on expression of angio-and fibrogenic factors,and cytokine concentrations during the healing process of human pressure ulcers[J].Int J Med Sci,2018,15(11):1105-1112.