急性肾损伤(acute kidney injury,AKI)是由多种病因导致的急性肾小球滤过率下降,以血清肌酐增高及尿量减少为主要表现的临床常见疾病。据统计,50%~70%的脓毒症患者会发生AKI,脓毒症是AKI首要致病原因,脓毒症相关性肾损伤的病死率明显高于其他原因的AKI病死率[1-4]。可溶性的黏附分子被认为是预测炎症严重程度和器官功能障碍的生物标志物[5-6]。内皮细胞黏附因子1(intercellular cell adhesion molecule-1,ICAM-1)及血管细胞黏附分子1(vascular cell adhesion molecule-1,VCAM-1)水平等黏附因子与脓毒症AKI的病死率显著相关[7-8],有研究显示,E选择素是脓毒症AKI早期诊断的独立预测因子[9]。动物试验表明,通过减少缺血后中性粒细胞向肾脏的浸润,阻断E选择素也可以预防发生急性肾衰竭[10]。超声下监测肾血管阻力指数可反应肾脏的血流和灌注变化,肾阻力指数(renal resistace index,RRI)的升高与脓毒症AKI发病风险和预后的相关[11],但是两者的相关性仍存在争议,有研究表明RRI升高与AKI的持续存在缺乏相关性,且受液体负荷等影响[12]。可溶性黏附因子被认为是脓毒症AKI的潜在生物标记物,但是黏附因子对于肾功能恢复的预测价值尚不明确,RRI可早期预测脓毒症AKI的发生和预后,但是受液体负荷等因素影响,鉴于此,本研究旨在探讨黏附因子联合超声监测RRI对脓毒症合并AKI患者的肾脏早期恢复的预测价值。
1 资料与方法
1.1 一般资料 选择2019年6月—2020年6月在河北医科大学第三医院重症医学科住院的脓毒症相关性AKI成年患者78例,年龄18~90岁,入选标准:①符合Sepsis 3.0诊断标准[13],存在明确的感染源,序贯器官衰竭评分(Sequential Organ Failure Assessment,SOFA)增加≥2分;②脓毒症引起的急性器官功能障碍或灌注不足的表现;③AKI诊断标准参照KDIGO分级[14]。
本研究经医院伦理委员会批准,所有研究对象均已签署入组的知情同意书。
1.2 肾功能恢复的标准 入组后30 d内,脱离肾替代治疗且肾功能恢复正常(肌酐及尿素氮在正常范围)。随访30 d,依据肾功能是否恢复,将研究对象进行分组为肾功能恢复组和未恢复组。
1.3 排除标准 年龄≤18周岁或孕妇;住ICU前已经存在基础慢性肾脏疾病或因慢性肾脏疾病需维持性血液透析者;接受肾移植等肾脏外科或者介入等手术或存在肾血管异常者;ICU住院时间≤3 d,拒绝参加本研究者。
1.4 研究方法
1.4.1 干预措施 对符合入选标准的研究对象,按拯救脓毒症运动指南(surviving sepsis campaign,SSC)进行液体复苏,必要时给予血管活性药物达到复苏目标,维持平均动脉压(mean artery pressure,MAP)≥65 mmHg(1 mmHg=0.133 kPa),上腔静脉血氧饱和度(central venous oxygen content,ScvO2)≥70%;当休克得到纠正后,按照限制性液体管理策略给予常规液体治疗。收集研究对象的一般资料,包括人口学资料(性别、年龄)及生命体征如收缩压、舒张压、MAP、尿量、实验室检验结果(血小板、白细胞、血降钙素原、血肌酐),感染部位、入ICU前后24 h时最大的急性生理与慢性健康状况评分Ⅱ(acute physiology and chronic health evaluation,APACHEⅡ)和SOFA评分,伴随的基础疾病、ICU住院时间、28 d病死率。观察终点为临床死亡,入组28 d以出现的临床死亡事件为观察终止时间。
1.4.2 黏附因子的测定 收集研究对象入ICU时及入ICU后1 d、3 d的血液标本4 mL、4 mL。其中一份血液标本15 min内进行离心后取血清及上清尿置于-80 ℃低温冰箱冻存待测,另一份血液标本送检验科检测肌酐及血常规及降钙素原等检查。采用酶联免疫吸附测定法检测第1 d和3 d的血清E-选择素(E-selectin,CD62E)、L-选择素(L-selectin,CD62L)、P-选择素(P-selectin,CD62P)、ICAM-1及VCAM-1水平(试剂盒购于Arigo Biolaboratories),血肌酐及白细胞及降钙素原(procalcitonin,PCT)等。
1.4.3 RRI测量 在研究对象入组后3 h内,由接受过超声系统培训且获得合格证的重症医学科医生对研究对象进行床旁超声检查,使用索诺声超声机的2~5 MHz凸阵超声探头测量。测量患者右肾,选取段动脉、叶间动脉或弓状动脉,在多普勒模式下测定血流速,取样容积为2~5 mm,每支动脉获取3~5个连续且频谱形态相似的流速波形,取均值作为该动脉的RI值。RI的计算公式:RI=[收缩期峰速(cm/s)-舒张末期血流速(cm/s)]/收缩期峰速(cm/s)。RI参考值为0.58~0.64。
1.5 统计学方法 应用SAS 9.4软件进行统计分析。计量资料两组间比较采用t检验。计数资料组间比较采用χ2检验或Fisher′s确切概率法;应用Logistic回归分析做关于AKI肾功能是或否恢复的危险因子筛选。采用Logistic回归模型做接受者操作特征(receiver operating characteristic,ROC)曲线分析黏附因子及RRI对于肾功能恢复的等指标的预测价值。P<0.05为差异有统计学意义。
2 结 果
2.1 脓毒症AKI患者肾功能恢复组与未恢复组的基线资料 共纳入脓毒症合并AKI患者78例,男性54例,女性24例,平均年龄(70.8±23.8)岁,肾功能恢复组30例,肾功能未恢复组48例,两组年龄及SOFA评分差异无统计学意义(P>0.05),早期恢复组的APACHEⅡ评分显著低于未恢复组,AKI早期肾功能恢复组的白细胞水平及PCT原均低于未恢复组(P=0.003),AKI早期肾功能恢复组的黏附因子(CD62P、CD62L、CD62E、ICAM-1、VCAM-1)表达均低于肾功能未恢复组,其中两组间VCAM-1表达差异有统计学意义(P=0.035),肾功能恢复组的病死率明显低于肾功能未恢复组(P<0.05))。见表1。
表1 脓毒症AKI肾功能恢复组与肾功能未恢复组的单因素分析
Table 1 Univariate analysis of renal function recovery and non renal function recovery in sepsis associated AKI![]()
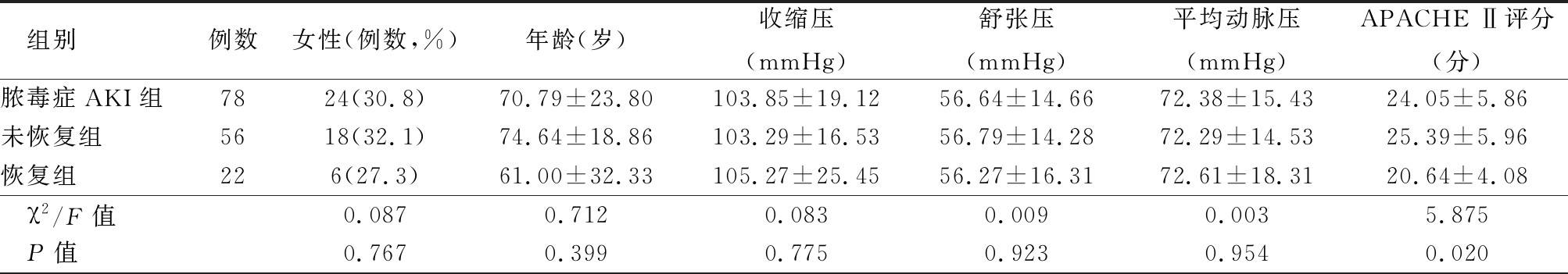
组别例数女性(例数,%)年龄(岁)收缩压(mmHg)舒张压(mmHg)平均动脉压(mmHg)APACHE Ⅱ评分(分)脓毒症AKI组7824(30.8)70.79±23.80103.85±19.1256.64±14.6672.38±15.4324.05±5.86未恢复组5618(32.1)74.64±18.86103.29±16.5356.79±14.2872.29±14.5325.39±5.96恢复组226(27.3)61.00±32.33105.27±25.4556.27±16.3172.61±18.3120.64±4.08 χ2/F值0.0870.7120.0830.0090.0035.875 P值0.7670.3990.7750.9230.9540.020
表1 (续)
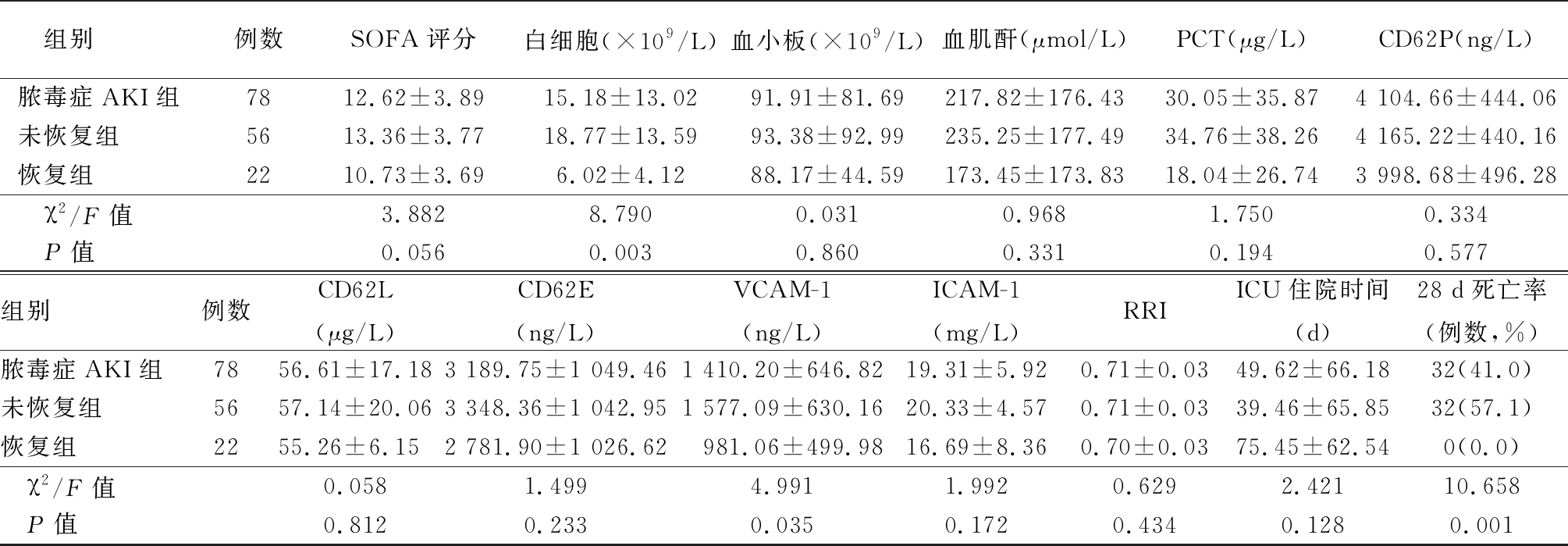
1 mmHg=0.133 kPa
组别例数SOFA评分白细胞(×109/L)血小板(×109/L)血肌酐(μmol/L)PCT(μg/L)CD62P(ng/L)脓毒症AKI组7812.62±3.8915.18±13.0291.91±81.69217.82±176.4330.05±35.874 104.66±444.06未恢复组5613.36±3.7718.77±13.5993.38±92.99235.25±177.4934.76±38.264 165.22±440.16恢复组2210.73±3.696.02±4.1288.17±44.59173.45±173.8318.04±26.743 998.68±496.28 χ2/F值3.8828.7900.0310.9681.7500.334 P值0.0560.0030.8600.3310.1940.577组别例数CD62L(μg/L)CD62E(ng/L)VCAM-1(ng/L)ICAM-1(mg/L)RRIICU住院时间(d)28 d死亡率(例数,%)脓毒症AKI组7856.61±17.183 189.75±1 049.461 410.20±646.8219.31±5.920.71±0.0349.62±66.1832(41.0)未恢复组5657.14±20.063 348.36±1 042.951 577.09±630.1620.33±4.570.71±0.0339.46±65.8532(57.1)恢复组2255.26±6.152 781.90±1 026.62981.06±499.9816.69±8.360.70±0.0375.45±62.540(0.0) χ2/F值0.0581.4994.9911.9920.6292.42110.658 P值0.8120.2330.0350.1720.4340.1280.001
2.2 黏附因子对脓毒症AKI预后的多因素分析 变量赋值如下,肾功能恢复(是=1,否=0),CD62L、CD62E、VCAM-1、ICAM-1及RRI均为连续性变量;应用Logistic回归分析,入组第1天的CD62P、CD62L、CD62E、VCAM-1及ICAM-1不是脓毒症AKI的预后独立影响因素(P>0.05)。见表2。
表2 黏附因子对脓毒症AKI预后的多因素分析
Table 2 Multivariate analysis of the effect of adhesive molecules on the prognosis of AKI with sepsis
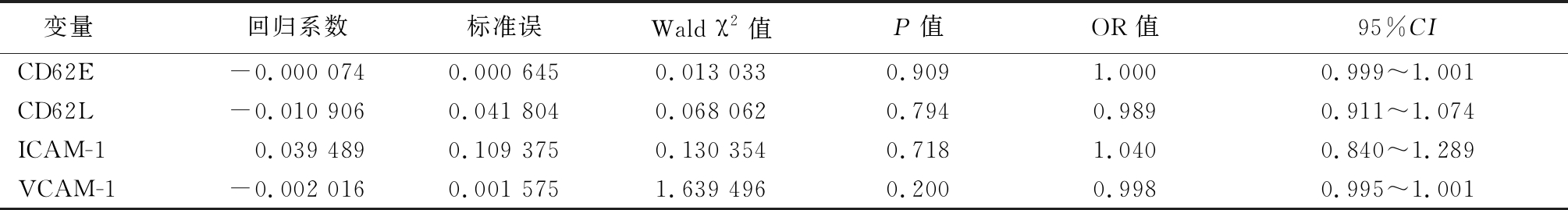
变量回归系数标准误Wald χ2值P值OR值95%CICD62E-0.000 0740.000 6450.013 0330.9091.0000.999~1.001CD62L-0.010 9060.041 8040.068 0620.7940.9890.911~1.074ICAM-10.039 4890.109 3750.130 3540.7181.0400.840~1.289VCAM-1-0.002 0160.001 5751.639 4960.2000.9980.995~1.001
2.3 黏附因子及RRI等对脓毒症AKI患者预测早期肾功能恢复的效能分析 应用ROC曲线分析,以RI=0.71为限值,预测脓毒症AKI患者早期肾功能恢复的敏感度为81.8%,特异度为71.4%,AUC=0.71;以CD62E>3 436 ng/L为限值,预测脓毒症AKI患者肾功能恢复的敏感度为85.7%,特异度为44.4%,AUC=0.63;以VCAM-1>1 233 ng/L为限值,预测脓毒症AKI患者肾功能恢复的敏感度为71.4%,特异度为77.8%,AUC=0.79;CD62E联合RI预测脓毒症患者肾功能恢复的敏感度为100%,特异度为17.9%,AUC=0.64;VCAM-1联合RI预测脓毒症患者肾功能恢复的敏感度为90.9%,特异度为32.1%,AUC=0.68。见表3。
表3 黏附因子及RRI等对脓毒症AKI患者预测早期肾功能恢复的效能分析
Table 3 Sensitivity and specificity values for adhesive molecules and RRI in the prediction of renal function recovery in septic acute kidney injury,by ROC curve analysis
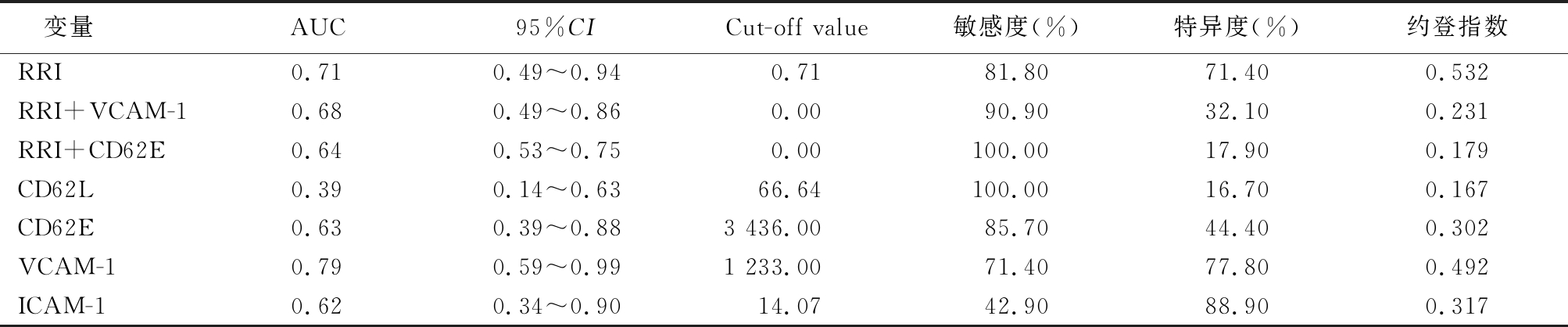
变量AUC95%CICut-off value敏感度(%)特异度(%)约登指数 RRI0.710.49~0.940.7181.8071.400.532RRI+VCAM-10.680.49~0.860.0090.9032.100.231RRI+CD62E0.640.53~0.750.00100.0017.900.179CD62L0.390.14~0.6366.64100.0016.700.167CD62E0.630.39~0.883 436.0085.7044.400.302VCAM-10.790.59~0.991 233.0071.4077.800.492ICAM-10.620.34~0.9014.0742.9088.900.317
2.4 黏附因子的动态变化对脓毒症AKI肾功能恢复的影响 在脓毒症AKI组,应用Logestic回归分析显示,入组第3天与第1天的CD62P、CD62L、CD62E及ICAM-1、VCAM-1的差值不是脓毒症AKI的肾功能恢复的独立影响因素(P>0.05)。见表4。
表4 黏附因子的动态变化对脓毒症AKI早期肾功能恢复的影响
Table 4 The relationship of changes of adhensive molecules and the renal function recovery in septic AKI
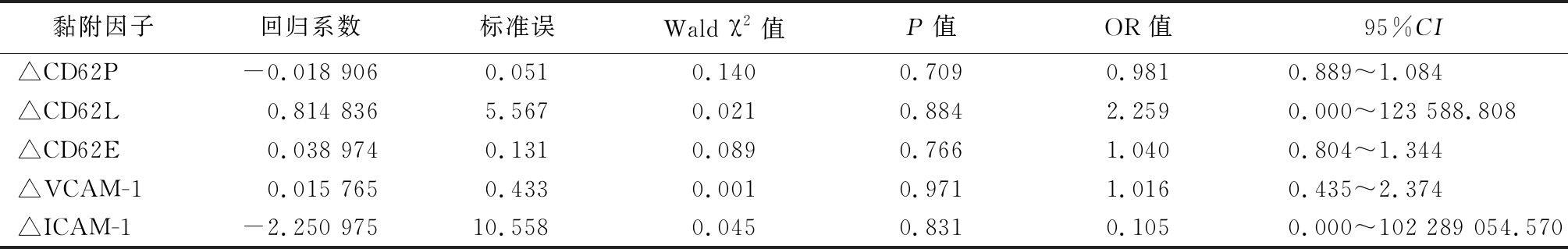
△CD62P:第3天与第1天的CD62P差值,△CD62L:第3天与第1天的CD62L差值,△CD62E:第3天与第1天的CD62E差值,△VCAM-1:第3天与第1天的VCAM-1差值,△ICAM-1:第3天与第1天的ICAM-1差值
黏附因子回归系数标准误Wald χ2值P值OR值95%CI△CD62P-0.018 9060.0510.1400.7090.9810.889~1.084 △CD62L0.814 8365.5670.0210.8842.2590.000~123 588.808△CD62E0.038 9740.1310.0890.7661.0400.804~1.344△VCAM-10.015 7650.4330.0010.9711.0160.435~2.374△ICAM-1-2.250 97510.5580.0450.8310.1050.000~102 289 054.570
3 讨 论
脓毒症相关性AKI是脓毒症及脓毒症休克的常见并发症,感染所引起的全身炎症反应是脓毒症AKI发生的基础,肾血流的变化和炎症反应的相互作用贯穿在AKI的发病早期及形成期。血管内皮细胞损伤和白细胞的激活、黏附、浸润在脓毒症疾病进展的过程中起了重要的作用,在白细胞的募集过程中,可溶性的黏附分子从细胞表面脱落,积聚在循环中。黏附因子包括E-选择素、L-选择素和P-选择素及ICAM-1及VCAM-1,其中E-选择素,L-选择素和P-选择素属于选择素在白细胞滚动中起作用,ICAM-1和VCAM-1属于免疫球蛋白域超家族细胞黏附分子,在牢固黏附和跨内皮的迁移起作用。有研究显示,Mac-1和LFA-1以及E-选择素和P-选择素参与了脓毒症诱导后肾脏中性粒细胞的募集[15-16],细胞黏附分子对中性粒细胞进入肾毛细血管丛是必不可少的,该过程是肾缺血-再灌注损伤的标志。本研究结果显示,在脓毒症AKI组,入组第3天的CD62E、CD62L表达水平较第1天下降,而入组第3天的ICAM-1、VACM-1表达水平较第1天升高,而ICAM-1、VACM-1在内皮迁移过程中起了重要的作用,进一步验证了炎症反应后内皮功能障碍在AKI发展中的作用,可溶性黏附因子是脓毒症AKI的潜在生物标志物,REGARDS队列研究显示,E-选择素与脓毒症AKI相关,但ICAM-1或VCAM-1与脓毒症相关的AKI风险无关[17],Su等[9]及姜伟等[18]研究发现,在脓毒症AKI患者中E-选择素表达水平显著高于脓毒症非AKI组,E-选择素表达水平是脓毒症肾损伤的独立危险因素。
肾功能恢复与脓毒性AKI患者的长期生存显著相关[19]。虽然,黏附因子对于脓毒症AKI早期诊断有较好的预测价值,但是,黏附因子对于AKI肾功能恢复的预测尚未得到很好的证实。本研究显示,肾功能恢复组的28 d病死率明显低于肾功能未恢复组,通过ROC曲线分析,以VCAM-1=1 233 ng/L为限值,可以预测脓毒症AKI患者肾功能早期恢复。国外有相似的研究报道,Kung等[8]研究显示,在脓毒症患者中,血清中VCAM-1和ICAM-1升高在急性脏器功能障碍的早期与多器官功能障碍相关,另有研究证实,第1天与第7天 ICAM-1及VCAM-1的变化是SOFA变化独立的预测因子[7]。关于预测脓毒症AKI肾功能恢复的临床危险因素仍无定论,Fiorentino等[19]研究显示,APACHE Ⅱ评分和基线SCr是脓毒性AKI恢复的独立的预测因素(AUC=0.79)。Demirjian等[20]学者发现由21个临床变量组成的ATN模型(Acute Renal Failure Trial Network)在预测病死率方面优于现有的疾病严重程度评分系统(AUC=0.85),此外,肾恢复的生物学标志物(BioMaRK)研究显示,在肾脏恢复(肾功能恢复定义为住院后60 d以内无肾替代治疗)和病死率的总体预测能力方面,基线临床模型和血浆生物标记物是相似的,由年龄、平均动脉压、机械通气、胆红素组成的临床模型联合血浆IL-8对于肾功能恢复及病死率的具有预测价值,肾脏恢复的AUC为0.76,死亡率AUC为0.78[21]。关于脓毒症AKI肾功能恢复的预测因子研究仍较少,黏附因子等新型生物标记物对于脓毒症AKI肾功能恢复的预测价值给脓毒症AKI的预后判断带来了新的研究方向。
分析RRI、VCAM-1及RRI联合VCAM-1对脓毒症AKI肾功能恢复的预测价值发现,单独应用一个指标时,以RRI的界值为0.71,VCAM-1的界值为1 233 ng/L,RRI的敏感性更好,VCAM-1的特异性较高。两个指标联合应用时,对AKI肾功能恢复的预测价值更大,敏感度升高为90.9%,特异度反而下降为32.1%,AUC=0.68。RRI仅反应了肾脏的皮质血流阻力情况,而VCAM-1反映了血管内皮功能,两者联合可提高敏感度,然而,由于在复苏的脓毒症动物和高动力循环状态的患者中,虽然肾脏血流不变或增加,脓毒症诱导的AKI仍然可以发生在肾充血环境中[22-23],两者联合时,特异度反而下降。多项临床研究显示,RRI在预测重症患者AKI转归,鉴别暂时性或持久性AKI方面有良好的应用价值[11],本研究结果与之相似。
综上所述,VCAM-I联合RRI对于脓毒症AKI患者的早期肾功能恢复有较高的预测价值,以RRI的界值为0.71,VCAM-1的界值为1 233 ng/L,可以作为对于脓毒症AKI患者的肾功能恢复的预测指标。
[1] Hoste EA,Bagshaw SM,Bellomo R,et al. Epidemiology of acute kidney injury in critically ill patients:the multinational AKI-EPI study[J]. Intensive Care Med,2015,41(8):1411-1423.
[2] Poston JT,Koyner JL.Sepsis associated acute kidney injury[J]. BMJ,2019,364:k4891.
[3] Peerapornratana S,Manrique-Caballero CL,Gómez H,et al.Acute kidney injury from sepsis:current concepts,epidemiology,pathophysiology,prevention and treatment[J].Kidney Int,2019,96(5):1083-1099.
[4] Skube SJ,Katz SA,Chipman JG,et al. Acute kidney injury and sepsis[J]. Surg Infect(Larchmt),2018,19(2):216-224.
[5] Gearing AJ,Newman W. Circulating adhesion molecules in disease[J]. Immunol Today,1993,14(10):506-512.
[6] Gearing AJ,Hemingway I,Pigott R,et al. Soluble forms of vascular adhesion molecules,E-selectin,ICAM-1,and VCAM-1:pathological significance[J]. Ann N Y Acad Sci,1992,667:324-331.
[7] Fang Y,Li C,Shao R,et al. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care:a prospective observational study[J]. Thromb Res,2018,171:149-154.
[8] Kung CT,Su CM,Chang HW,et al. Serum adhesion molecules as outcome predictors in adult severe sepsis patients requiring mechanical ventilation in the emergency department[J]. Clin Biochem,2014,47(15):38-43.
[9] Su CM,Cheng HH,Hung CW,et al. The value of serial serum cell adhesion molecules in predicting acute kidney injury after severe sepsis in adults[J]. Clin Chim Acta,2016,457:86-91.
[10] Kato N,Yuzawa Y,Kosugi T,et al. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion[J]. J Am Soc Nephrol,2009,20(7):1565-1576.
[11] Darmon M,Schortgen F,Vargas F,et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients[J]. Intensive Care Med,2011,37(1):68-76.
[12] Darmon M,Bourmaud A,Reynaud M,et al. Performance of Doppler-based resistive index and semi-quantitative renal perfusion in predicting persistent AKI:results of a prospective multicenter study[J]. Intensive Care Med,2018,44(11):1904-1913.
[13] Singer M,Deutschman CS,Seymour CW,et al. The third international consensus definitions for sepsis and septic shock(Sepsis-3)[J]. JAMA,2016,315(8):801-810.
[14] Khwaja A. KDIGO clinical practice guidelines for acute kidney injury[J]. Nephron Clin Pract,2012,120(4):c179-184.
[15] Herter JM,Rossaint J,Spieker T,et al. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury[J]. J Innate Immun,2014,6(5):597-606.
[16] Wang YM,Han RL,Song SG,et al. Inhibition of PARP overactivation protects acute kidney injury of septic shock[J]. Eur Rev Med Pharmacol Sci,2018,22(18):6049-6056.
[17] Powell TC,Powell SL,Allen BK,et al. Association of inflammatory and endothelial cell activation biomarkers with acute kidney injury after sepsis[J]. Springerplus,2014,3:207.
[18] 蒋伟,张健锋,李山峰,等.血清细胞黏附分子对脓毒症急性肾损伤的预测价值研究[J].中国全科医学,2020,23(20):2525-2529.
[19] Fiorentino M,Tohme FA,Wang S,et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery[J]. PLoS One,2018,13(6):e0198269.
[20] Demirjian S,Chertow GM,Zhang JH,et al. VA/NIH acute renal failure trial network. model to predict mortality in critically ill adults with acute kidney injury[J]. Clin J Am Soc Nephrol,2011,6(9):2114-2120.
[21] Pike F,Murugan R,Keener C,et al.Biological markers for recovery of kidney(BioMaRK) study investigators. biomarker enhanced risk prediction for adverse outcomes in critically Ill patients receiving RRT[J]. Clin J Am Soc Nephrol,2015,10(8):1332-1339.
[22] Kellum JA,Ronco C,Vincent JL. Unveiling current controversies in acute kidney injury[J]. Contrib Nephrol,2011,174:1-3.
[23] Bouglé A,Duranteau J. Pathophysiology of sepsis-induced acute kidney injury:the role of global renal blood flow and renal vascular resistance[J]. Contrib Nephrol,2011,174:89-97.