心血管疾病现已成为全球首位死亡原因[1],动脉粥样硬化、冠心病、心肌梗死、高血压以及心力衰竭等心血管疾病给人类造成了巨大的健康和经济负担。外周血管病冠状动脉疾病患者血浆5-羟色胺(5-hydroxytryptamin,5-HT)水平升高,提示5-HT在心血管疾病的发生发展中发挥作用[2]。研究表明,5-HT主要是通过5-HT受体介导血管内皮功能障碍,造成血管收缩、促进血小板活化和聚集、血栓形成以及血管平滑肌细胞增殖与迁移等病理生理过程,促进心血管疾病的发生发展。近年来,5-HT受体拮抗剂以及5-HT再摄取抑制剂(selective serotonin reuptake inhibitors,SSRIs)用于治疗心血管疾病,但临床结果尚不一致,研究仍在进行中。笔者就5-HT在心血管疾病中的作用机制及5-HT受体拮抗剂和再摄取抑制剂在心血管疾病中的研究现状进行综述。
1 概述
5-HT是一种广泛存在于生物体内的血管活性物质。超过90%的5-HT在肠道合成,主要由肠嗜铬样细胞(enterochromaffin-like cells,ECs)、黏膜肥大细胞和肌间神经元合成。血小板可从肠道主动摄取5-HT,在血小板中储存的5-HT还在止血过程中发挥重要作用。
5-HT是色氨酸在色氨酸羟化酶(tryptophan hydroxylase,TPH)的作用下首先生成5-羟色氨酸(5-hydroxytryptophane,5-HTP),进一步在5-羟色氨酸脱羧酶(5-hydroxytryptophan decarboxylase,5-HTPDC)的作用下合成的[3]。5-HT的代谢主要通过膜转运体再摄取和酶解两种方式进行,膜转运体摄取依赖Na+/Cl-依赖型转运体,5-HT被膜转运体摄入包浆,再经囊泡单胺类转运体进入囊泡内储存;酶解途径是5-HT在单胺氧化酶(monamine oxidase,MAO)的作用下成为5-羟吲哚乙醛,再在醛脱氢酶作用下成为5-羟吲哚乙酸(5-hydroxyindoleacetic acid,5-HIAA)。
目前,根据受体功能活动状态以及信号传导方式,将5-HT受体分为7个类型和18个亚型。其中5-HT3受体是一种配体门控离子通道,可以转运Na+、K+和Ca2+[4],其他的亚型均为G蛋白偶联受体,每个受体的信号传导途径各不相同(表1)。研究显示,分布在心血管系统的受体有5-HT1B、5-HT1D、5-HT2、5-HT2B、5-HT4、5-HT7,介导一系列心血管效应。
表1 5-HT受体家族
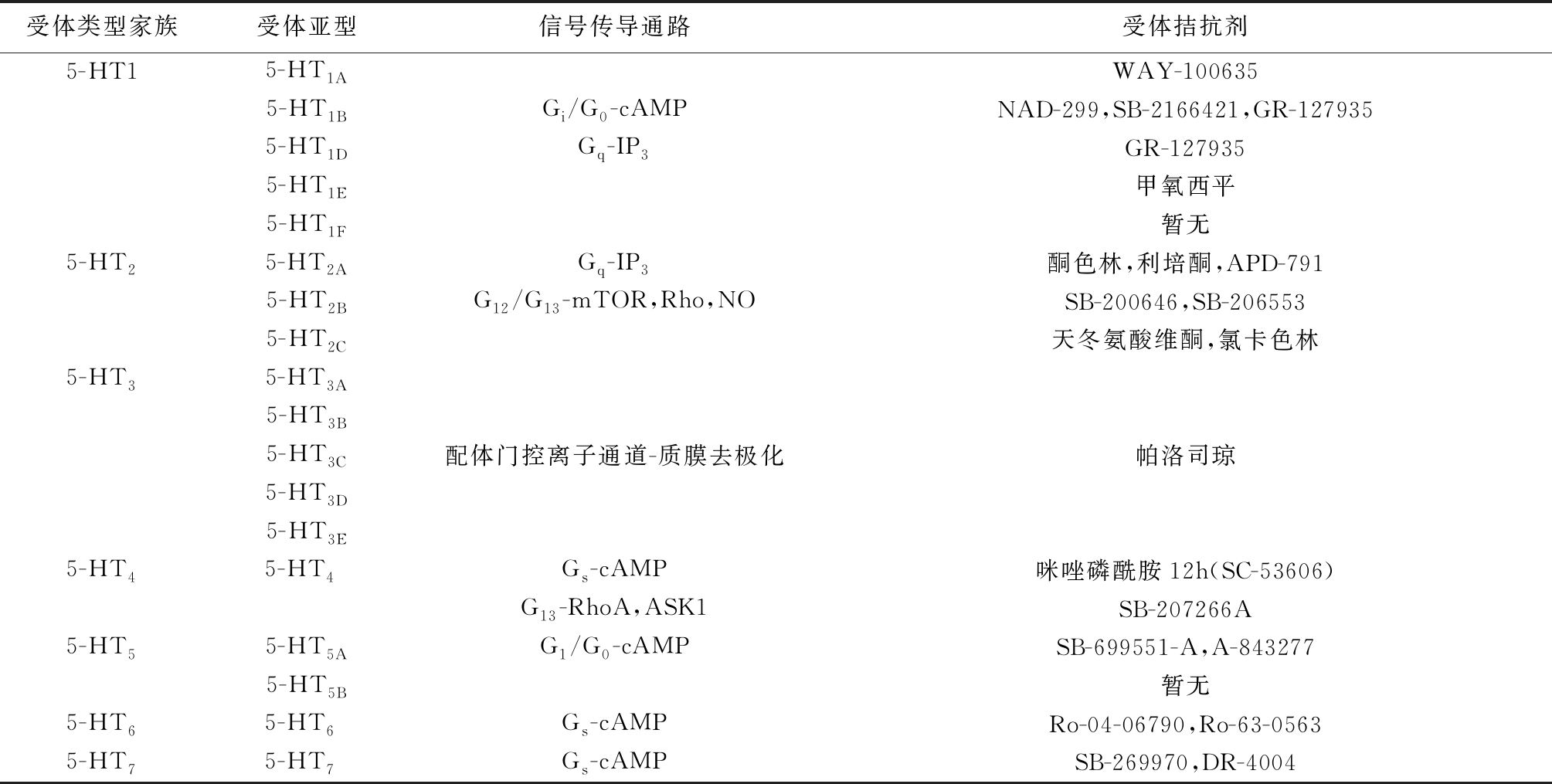
受体类型家族受体亚型信号传导通路受体拮抗剂5-HT15-HT1AWAY-1006355-HT1BGi/G0-cAMPNAD-299,SB-2166421,GR-1279355-HT1DGq-IP3GR-1279355-HT1E甲氧西平5-HT1F暂无5-HT25-HT2AGq-IP3酮色林,利培酮,APD-7915-HT2BG12/G13-mTOR,Rho,NOSB-200646,SB-2065535-HT2C天冬氨酸维酮,氯卡色林5-HT35-HT3A5-HT3B5-HT3C配体门控离子通道-质膜去极化帕洛司琼5-HT3D5-HT3E5-HT45-HT4 Gs-cAMP咪唑磷酰胺12h(SC-53606)G13-RhoA,ASK1SB-207266A5-HT55-HT5AG1/G0-cAMPSB-699551-A,A-8432775-HT5B暂无5-HT65-HT6 Gs-cAMPRo-04-06790,Ro-63-05635-HT75-HT7 Gs-cAMPSB-269970,DR-4004
2 5-HT与心血管疾病
2.1 5-HT与动脉粥样硬化 5-HT主要通过以下机制促进动脉粥样硬化的发生发展:①血管内皮损伤后介导血管收缩,对49例动脉粥样硬化患者进行5-HT水平以及血管内皮功能等检查,结果显示,血浆5-HT水平与早期动脉粥样硬化患者的血管内皮损伤呈正相关[5]。在兔冠状动脉粥样硬化模型中,冠状动脉内皮损伤后,5-HT可通过5-HT1B受体诱导冠脉痉挛[6]。②介导血小板活化、聚集及血栓形成,血管内皮损伤后,血小板聚集活化并释放5-HT等生物活性物质,5-HT通过血小板表面的5-HT2A及5-HT3受体,促进血小板聚集,引起血管收缩[7]。Kawano等[8]认为5-HT通过5-HT2A受体增强促凝血活性的同时,还降低纤溶活性,从而促进血管损伤部位血栓形成。选择性5-HT2A受体拮抗剂APD-791可以改善冠状动脉通畅性,并且可能成为抗血小板治疗的替代或辅助疗法。③介导血管平滑肌细胞增殖、迁移以及表型转化,5-HT是参与动脉粥样硬化中血管平滑肌细胞异常增殖的信号分子之一。程茹等[9]认为,5-HT通过5-HT2A /G蛋白/Src家族/PTK-PKC-MAPK通路,不仅可以促进血管平滑肌细胞有丝分裂,还可以通过上调胆固醇酰基转移酶1(acyl-coenzyme A: cholesterol acyltransferase-1,ACAT-1)促进巨噬细胞源性泡沫细胞形成,促进动脉粥样硬化斑块的进展;单核细胞趋化蛋白1(monocyte chemotactic protein-1,MCP-1)激活JAK2/STAT3通路可增强5-HT诱导的血管平滑肌细胞有丝分裂;AngⅡ和5-HT分别通过作用于AT1和5-HT2A受体在诱导血管平滑肌细胞增殖中起协同作用,MAPK和JAK/STAT通路的激活可能与AngⅡ和5-HT之间的协同作用有关;低密度脂蛋白(low density lipoprotein,LDL)、氧化低密度脂蛋白(oxidized low density lipoprotein,ox-LDL)、溶血磷脂酰胆碱( lyso-phosphatidylcholine,LPC)、H2O2及主要脂质过氧化产物4-羟基-2-壬烯醛(4-hydroxy-2-nonenaldehyde,HNE)与5-HT也具有协同作用,共同诱导血管平滑肌细胞增殖,促进动脉粥样硬化斑块的形成。④介导炎症、氧化应激,5-HT可以通过调控炎性细胞因子及机体氧化应激水平促进动脉粥样硬化的发生发展。5-HT作用于5-HT2受体,通过PKC依赖途径促进血管平滑肌细胞IL-1b/IL-6的合成,提示5-HT参与了动脉粥样硬化形成过程中的血管炎性反应。Sugiura等[10]通过检测132例有心血管危险因素患者的血浆血小板计数、全血5-HT水平以及活性氧代谢产物(diacron reactive oxygen metabolites,d-ROM)结果显示,氧化应激可通过释放5-HT促进血小板活化,进而促进血管炎症和动脉粥样硬化的发展。同时,5-HT代谢产物5-HIAA也被证明介导氧化应激促进动脉粥样硬化的发生发展[11]。
临床研究显示,选择性5-HT2A受体拮抗剂盐酸沙格雷酯可显著降低动脉粥样硬化斑块的大小和泡沫细胞的数量,联合应用阿司匹林治疗6个月可以减少2型糖尿病患者轻至中度冠状动脉粥样硬化斑块的体积,这些有益的效果归因于盐酸沙格雷酯的抗炎以及胰岛素增敏特性[12]。相关研究认为,盐酸沙格雷酯可通过上调内皮一氧化氮合酶(endothelial nitric oxide synthase,eNOS),降低血液黏度、氧化应激、血脂以及抑制平滑肌细胞和巨噬细胞的增殖延缓动脉粥样硬化的进展[13]。此外,一项前瞻性研究的初步结果表明,盐酸沙格雷酯可预防股动脉支架植入术后再狭窄[11]。综上所述,盐酸沙格雷酯可能通过保护内皮细胞、减少血小板聚集、预防血栓形成以及减少胆固醇和巨噬细胞向血管内迁移而在预防动脉粥样硬化方面发挥有益作用。
2.2 5-HT与高血压 5-HT可升高总外周循环阻力,被认为是高血压的致病因素之一[14]。在多年的研究中,许多学说支持5-HT及受体在高血压的发病和维持中起一定作用[15]。然而至今尚未有一种完备的理论能够阐明5-HT在不同血压状态、物种、部位血管中的作用以及对血压的具体影响。
将5-HT快速静脉注射到麻醉的啮齿动物后,可观察到血压的三相反应:降压反应,被归因于激活Bezold-Jarisch反射导致心率减慢;短暂的血压显著升高,被认为是由5-HT2受体介导的;缓慢降压反应,归因于5-HT1B/1D和5-HT7受体的共同激活。
长时间外源性给予5-HT,多表现为降压作用。有报道雄性SD大鼠服用5-HT后至少出现两个降压期:给药后3 h内和给药后近20 h,且降压效应更为稳定[14];亦可见5-HT长期应用使醋酸脱氧皮质酮(desoxycortone acetate,DOCA)-盐型高血压大鼠的血压下降超过50 mmHg(1 mmHg=0.133 kPa),并在一周内保持较低的血压[16]。这些研究表明5-HT在降低啮齿动物血压中发挥作用。有研究报道这种降压效应与一氧化氮合酶(nitric oxide synthase,NOS)和5-羟色胺转运体(serotonin transporter,SERT)的作用有关:①5-HT可介导NOS促进不同类型的细胞和组织生成一氧化氮而降低血压,同时NOS又可阻碍5-HT被SERT摄取从而负反馈调控5-HT[17],而NOS抑制剂L-硝基精氨酸(NG-nitro-L-arginine,L-NNA)可取消5-HT引起的降压作用并升高血压[18]。②SERT功能对血管内5-HT的浓度调节起重要作用,而SERT功能障碍小鼠由5-HT引起的血压下降幅度相比于SERT功能正常小鼠减少了50%[16],说明5-HT的降压作用可能是浓度依赖性的。
5-HT作用于中枢及外周对血压影响的研究,显示5-HT对于血压的影响更加复杂。5-HT的关键合成酶色氨酸羟化酶(tryptophan hydroxylase,TPH)分外周型(tryptophan hydroxylase-1,TPH1)和中枢型(tryptophan hydroxylase-2,TPH2)。已经建立了TPH1基因敲除小鼠,其中枢5-HT水平正常。测量氟烷麻醉状态下的TPH1基因敲除小鼠的动脉血压(颈动脉插管),其基础动脉压显著高于野生型小鼠[19]。这意味着外周5-HT可起到降低血压的作用,或者抑制了一种升高血压的机制。而在TPH2基因敲除小鼠中,5-HT的缺失主要在中枢神经系统,外周5-HT基本正常。TPH2基因敲除小鼠的血压显著低于野生型,特别是在傍晚和夜间[20],这表明中枢5-HT有着升高血压的潜力。选择性中枢5-HT2A受体介导动脉血压升高[21-22]以及TPH抑制剂PCPAME(400 mg/kg)能显著降低雄性DOCA-盐型高血压大鼠的血压,且脑干5-HT耗竭实验结果[23]亦佐证了这一观点。
目前的理解是,即使外周5-HT似乎不是引起高血压的主要原因,5-HT也会加剧这种疾病的伴随现象,如血小板聚集、血栓栓塞症并发症的风险、动脉粥样硬化造成的血管损伤等[24]。而高血压患者循环中游离5-HT增多,故不难理解改变血浆5-HT浓度在预防或改善高血压方面的潜力。氟西汀是SSRIs,早有报道氟西汀降低自发性高血压大鼠血压。5-HT/去甲肾上腺素再摄取抑制剂(serotonin/norepinephrine reuptake inhibitor,SNRIs)如文拉法辛和度洛西汀亦发现了降压作用[25]。但是结论尚存争议[26]。
2.3 5-HT与心肌梗死 高浓度5-HT所致的血小板活化、聚集和病变冠状动脉收缩会显著增加心肌梗死等心血管事件的发生率[27]。同时,心肌梗死也可促进血小板活化,进一步释放5-HT,加重心肌缺血,促进心肌的再灌注损伤[28]。相关研究认为,长期服用SSRIs可以耗竭血小板5-HT进而降低心肌梗死风险[29]。
SSRIs通过抑制5-HT再摄取转运体介导的血小板摄取耗竭外周5-HT。但目前临床研究关于SSRIs的潜在心血管效应仍存在争议。一方面,一些临床数据显示服用SSRIs的患者心血管风险没有增加,甚至更低[30-31]。而另一些临床研究表明SSRIs的摄入与发生心血管事件的更高风险之间存在联系[32-33]。Rami等[34]用氟西汀治疗载脂蛋白E缺陷小鼠发现可以促进动脉粥样硬化发生发展,而抑制TPH1并不促进动脉粥样硬化,表明氟西汀的致动脉粥样硬化作用不依赖于外周5-HT的耗竭,而是通过增强髓系趋化因子CCL5介导的β2整合素结合能力和白细胞与血管内皮细胞间的相互作用这两种5-HT非依赖的方式促进动脉粥样硬化。同时,氟西汀还可以增强血管通透性,使白细胞的跨内皮细胞迁移更加容易。Schumacher等[35]在心肌梗死后心力衰竭的小鼠模型中证明,SSRIs类药物帕罗西汀选择性抑制G蛋白偶联受体激酶2(G protein-coupled receptor kinase-2,GRK2)进而改善心肌梗死后心脏的功能和结构,预防心力衰竭的发展。关于SSRIs的潜在心血管效应及其作用机制仍然有待进一步的研究,这将对并发心血管疾病抑郁症患者的治疗干预提供更加明确的思路。
2.4 5-HT与肺动脉高压 肺动脉高压是指肺动脉压升高并且>25 mmHg的疾病,以血管收缩、增生、纤维化和炎症为特征。有研究表明,5-HT主要通过5-HT1B和5-HT2A受体介导肺动脉收缩及SERT依赖的血管平滑肌增殖[36],最终导致肺动脉高压。在不缺氧大鼠中,5-HT诱导的肺动脉收缩主要是5-HT2A受体介导;而在慢性缺氧的大鼠中,5-HT诱导的肺血管收缩是5-HT2A和5-HT1B受体共同介导[37]。5-HT诱导肺动脉平滑肌细胞有丝分裂是由丝裂原活化蛋白激酶(mitogen activated protein kinase,MAPK)依赖的途径介导[38]。同时,肺动脉高压时平滑肌细胞的增生一定程度上可归因于SERT过度表达[39],MicroRNA-361-3p过度表达通过降低SERT水平抑制5-HT诱导的肺动脉血管平滑肌细胞增殖[40]。然而也有文献支持5-HT2B受体在猪和人的肺动脉的内皮细胞和平滑肌细胞表达,并且在原发性肺动脉高压患者的肺动脉中高度表达[41]。5-HT2B受体的激活会直接激活eNOS,从而导致血管平滑肌的舒张。
5-HT2A受体拮抗剂酮色林调节肺动脉高压小鼠中MAPK/Akt信号的转导,从而恢复正常的肺血管发育并防止肺动脉高压与支气管肺发育不良等肺血管疾病的发生[42]。LY393558拮抗5-HT1B和5-HT2A受体,进而抑制肺动脉平滑肌的增殖和收缩,预防并逆转肺动脉高压和重塑[34]。5-HT2受拮抗剂特麦角脲[43]拮抗 5-HT引起的肺血管收缩,并且抑制肺动脉血管平滑肌细胞的增殖,进而阻止大鼠肺动脉高压的发生发展。此外,特麦角脲还减轻炎症并抑制右心室肥厚和纤维化,改善右心室的收缩功能,进而预防右心衰竭[44]。
2.5 5-HT与其他心血管疾病
2.5.1 心脏瓣膜病 心脏瓣膜病指瓣膜由于各种病理变化而影响血液的正常流动,从而导致心脏功能的异常,最终导致心力衰竭的一种病变。5-HT2B受体在心瓣膜上表达,对心脏发育和功能起维持作用[45]。当血浆5-HT过多或者5-HT2B受体在心瓣膜上表达过多时,会导致瓣膜叶增厚、舒张期关闭不全、肺动脉高压,最终形成心力衰竭等一系列变化[46]。在胃肠道发生类癌时,其作为神经内分泌肿瘤会分泌大量的5-HT,致使大约一半的患者出现“类癌心脏病”,其特征是二尖瓣和三尖瓣的萎缩性病变[47]。大量实验和临床随访观察在运用5-HT受体激动剂治疗神经系统疾病和肥胖时,不可忽视其心脏瓣膜毒性[48]。
2.5.2 心肌重构 以高血压为代表的后负荷长期增高会导致心肌肥厚、纤维化和心肌重构。Ayme-Dietrich等[49]研究显示,5-HT2A受体在高血压引起的左心室肥厚中表达增加。在人衰竭的心脏中,相比于5-HT2B受体表达水平很低的周围正常组织,5-HT2B受体明显过度表达。同样,Marzak等[50]通过研究5-HT2B慢性受体拮抗剂RS-127445对老年自发性高血压大鼠的影响,显示阻断5-HT2B受体并不能减轻心肌肥厚。5-HT2B受体对心肌细胞和心肌成纤维细胞有着复杂的作用机制。
2.5.3 心力衰竭 5-HT2A和5-HT4受体表达显著上调[51],并通过不同机制介导变力反应:5-HT2A受体信号类似于α1-肾上腺素受体,通过增强肌球蛋白轻链磷酸化[52]导致Ca2+敏化,从而引起变力作用。5-HT4受体信号类似于β-肾上腺素受体,通过cAMP/PKA/Ca2+转运蛋白通路引起变力作用[53]。而到目前为止,所有使用cAMP增强剂治疗慢性心力衰竭的临床试验,包括磷酸二酯酶抑制剂,均对心力衰竭患者存活率产生不利影响[54]。有研究表明盐酸沙格雷酯阻断5-HT2A受体可阻止5-HT2A/PLC通路的激活,抑制细胞内Ca2+的过度释放[55],阻止了5-HT引起的血管平滑肌细胞内Ca2+升高和细胞增殖[56],且在冠状动脉平滑肌细胞上也有类似的作用,被证明可以减轻大鼠急性心肌梗死引起的心律失常和心功能障碍[57]。而Kjekshus等[58]的研究表明,慢性心力衰竭患者使用5-HT4受体拮抗剂派波色罗治疗24周后,心力衰竭有了轻微而有意义的改善。进一步证明了5-HT及5-HT2A和5-HT4受体在心力衰竭的发生发展中的重要地位及其作为一治疗靶点的潜力。
3 结语和展望
综上所述,5-HT与其受体结合后,可以发挥包括介导血管内皮功能障碍、调控血小板活化聚集、血栓形成、血管收缩以及血管平滑肌细胞增殖和迁移等一系列病理生理效应,在动脉粥样硬化、高血压、心肌梗死、心肌肥厚、肺动脉高压以及心力衰竭等心血管疾病中发挥重要作用。目前盐酸沙格雷酯、特麦角脲等5-HT受体拮抗剂以及氟西汀、帕罗西汀等SSRIs已被用于临床治疗心血管疾病,但对某些药物的心血管效应的研究仍有争议。尽管5-HT在心血管疾病中具体的作用机制尚未阐明,但随着医学科研及制药水平的发展,针对5-HT相关通路靶点的新药物将会被研究开发并应用于临床,为心血管疾病的治疗提供新方法和新思路。
[1] GBD 2016 Causes of Death Collaborators.Global,regional,and national age-sex specific mortality for 264 causes of death,1980-2016:a systematic analysis for the Global Burden of Disease Study 2016[J]. Lancet,2017,390(10100):1151-1210.
[2] Vikenes K,Farstad M,Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events[J]. Circulation,1999,100(5):483-489.
[3] Walther DJ,Peter JU,Bashammakh S,et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform[J]. Science,2003,299(5603):76.
[4] Göthert M,Bönisch H,Malinowska B,et al. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part II. Some contributions of Manfred Göthert[J]. Pharmacol Rep,2020,72(2):271-284.
[5] Sugiura T,Dohi Y,Yamashita S,et al. Analytical evaluation of plasma serotonin and sphingosine 1-phosphate and their clinical assessment in early atherosclerosis[J]. Coron Artery Dis,2012,23(4):234-238.
[6] Ishida T,Kawashima S,Hirata K,et al. Serotonin-induced hypercontraction through 5-hydroxytryptamine 1B receptors in atherosclerotic rabbit coronary arteries [J]. Circulation,2001,103(9):1289-1295.
[7] Fraer M,Kilic F. Serotonin:a different player in hypertension-associated thrombosis[J]. Hypertension,2015,65(5):942-948.
[8] Kawano H,Tsuji H,Nishimura H,et al. Serotonin induces the expression of tissue factor and plasminogen activator inhibitor-1 in cultured rat aortic endothelial cells[J]. Blood,2001,97(6):1697-1702.
[9] 程茹,傅继华.外周5-羟色胺在动脉粥样硬化中的作用研究进展[J].药学研究,2017,36(5):283-286.
[10] Sugiura T,Dohi Y,Yamashita S,et al. Serotonin in peripheral blood reflects oxidative stress and plays a crucial role in atherosclerosis:Novel insights toward holistic anti-atherothromboticstrategy[J]. Atherosclerosis,2016,246:157-160.
[11] Kato Y,Oki K,Suga N,et al. A novel quinone derived from 5-hydroxyindoleacetic acid reacts with protein:Possible participation of oxidation of serotonin and its metabolite in the development of atherosclerosis[J]. Free Radic Biol Med,2016,101:500-510.
[12] Lee DH,Chun EJ,Hur JH,et al. Effect of sarpogrelate,a selective 5-HT receptor antagonist,on characteristics of coronary artery disease in patients with type 2 diabetes[J].Atherosclerosis,2017,257:47-54.
[13] Xu YJ,Zhang M,Ji L,et al. Suppression of high lipid diet induced by atherosclerosis sarpogrelate[J]. J Cell Mol Med,2012,16(10):2394-2400.
[14] Banes AK,Watts SW. Arterial expression of 5-HT2B and 5-HT1B receptors during development of DOCA-salt hypertension[J]. BMC Pharmacol,2003,3:12.
[15] Watts SW,Morrison SF,Davis RP,et al. Serotonin and blood pressure regulation[J]. Pharmacol Rev,2012,64(2):359-388.
[16] Patrick Davis R,Linder AE,Watts SW. Lack of the serotonin transporter(SERT) reduces the ability of 5-hydroxytryptamine to lower blood pressure[J]. Naunyn Schmiedebergs Arch Pharmacol,2011,383(5):543-546.
[17] Chanrion B,Mannoury la Cour C,Bertaso F,et al. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity[J]. Proc Natl Acad Sci U S A,2007,104(19):8119-8124.
[18] Tan T,Watts SW,Davis RP. Drug delivery:enabling technology for drug discovery and development. iprecio micro infusion pump:programmable,refillable,and implantable[J].Front Pharmacol,2011,2:44.
[19] Morecroft I,Dempsie Y,Bader M,et al. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension[J]. Hypertension,2007,49(1):232-236.
[20] Alenina N,Kikic D,Todiras M,et al. Growth retardation and altered autonomic control in mice lacking brain serotonin[J]. Proc Natl Acad Sci U S A,2009,106(25):10332-10337.
[21] Wang H,Gao XY,Rao F,et al. Mechanism of contractile dysfunction induced by serotonin in coronary artery in spontaneously hypertensive rats[J]. Naunyn Schmiedebergs Arch Pharmacol,2020,393(11):2165-2176.
[22] Watanabe S,Matsumoto T,Ando M,et al. Multiple activation mechanisms of serotonin-mediated contraction in the carotid arteries obtained from spontaneously hypertensive rats[J]. Pflugers Arch,2016,468(7):1271-1282.
[23] Ni W,Lookingland K,Watts SW.Arterial 5-hydroxytryptamine transporter function is impaired in deoxycorticosterone acetate and Nomega-nitro-L-arginine but not spontaneously hypertensive rats[J]. Hypertension,2006,48(1):134-140.
[24] Fraer M,Kilic F. Serotonin:a different player in hypertension-associated thrombosis[J]. Hypertension,2015,65(5):942-948.
[25] Chudasama HP,Bhatt PA. Evaluation of anti-obesity activity of duloxetine in comparison with sibutramine along with its anti-depressant activity:an experimental study in obese rats[J]. Can J Physiol Pharmacol,2009,87(11):900-907.
[26] Shelton RC. Serotonin and norepinephrine reuptake inhibitors[J]. Handb Exp Pharmacol,2019,250:145-180.
[27] O′Neil A,Fisher AJ,Kibbey KJ,et al. The addition of depression to the framingham risk equation model for predicting coronary heart disease risk in women[J]. Prev Med,2016,87:115-120.
[28] Pasalic L,Wang SS,Chen VM. Platelets as biomarkers of coronary artery disease[J]. Semin Thromb Hemost,2016,42(3):223-233.
[29] Kim Y,Lee YS,Kim MG,et al. The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events:a meta-analysis of randomized-controlled studies in depression[J]. Int Clin Psychopharmacol,2019,34(1):9-17.
[30] He Y,Cai Z,Zeng S,et al. Effect of fluoxetine on three-year recurrence in acute ischemic stroke:a randomized controlled clinical study[J]. Clin Neurol Neurosurg,2018,168:1-6.
[31] Coupland C,Hill T,Morriss R,et al. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64:cohort study using primary care database[J]. BMJ,2016,352:1350.
[32] Biffi A,Scotti L,Corrao G. Use of antidepressants and the risk of cardiovascular and cerebrovascular disease:a meta-analysis of observational studies[J]. Eur J Clin Pharmacol,2017,73(4):487-497.
[33] Rieckmann N,Kronish IM,Shapiro PA,et al. Serotonin reuptake inhibitor use,depression,and long-term outcomes after an acute coronary syndrome:a prospective cohort study[J]. JAMA Intern Med,2013,173(12):1150-1151.
[34] Rami M,Guillamat-Prats R,Rinne P,et al. Chronic intake of the selective serotonin reuptake inhibitor fluoxetine enhances atherosclerosis[J]. Arterioscler Thromb Vasc Biol,2018,38(5):1007-1019.
[35] Schumacher SM,Gao E,Zhu W,et al. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction[J]. Sci Transl Med,2015,7(277):277ra31.
[36] Baranowska-Kuczko M,Koziowska H,Schlicker E,et al. Reduction of the serotonin 5-HT and 5-HT receptor-mediated contraction of human pulmonary artery by the combined 5-HT receptor antagonist and serotonin transporter inhibitor LY393558[J]. Pharmacol Rep,2020,72(3):756-762.
[37] Wallace E,Morrell NW,Yang XD,et al. A Sex-Specific MicroRNA-96/5-Hydroxytryptamine 1B Axis Influences Development of Pulmonary Hypertension[J]. Am J Respir Crit Care Med,2015,191(12):1432-1442.
[38] 王玉秀,徐兴祥,迟焙元,等.5-羟色胺在肺动脉高压中作用机制的研究进展[J].中华肺部疾病杂志,2019,12(3):363-367.
[39] Jiang X,Yuan L,Li P,et al. Effect of Simvastatin on 5-HT and 5-HTT in a Rat Model of Pulmonary Artery Hypertension[J]. Cell Physiol Biochem,2015,37(5):1712-1724.
[40] Zhang Y,Chen Y,Chen G,et al. Upregulation of miR-361-3p suppresses serotonin-induced proliferation in human pulmonary artery smooth muscle cells by targeting SERT[J]. Cell Mol Biol Lett,2020,25:45.
[41] Launay JM,Herve P,Peoc′h K,et al. Function of the 5-hydroxytryptamine 2B receptor in pulmonary hypertension[J]. Nat Med,2002,8(10):1129-1135.
[42] Delaney C,Sherlock L,Fisher S,et al. Serotonin 2A receptor inhibition protects against the development of pulmonary hypertension and pulmonary vascular remodeling in neonatal mice[J]. Am J Physiol Lung Cell Mol Physiol,2018,314(5):L871-L881.
[43] Alc ntara-V
ntara-V zquez O,Villamil-Hern
zquez O,Villamil-Hern ndez MT,S
ndez MT,S nchez-López A,et al. Blocking properties of terguride at the 5-HT2 receptor subtypes mediating cardiovascular responses in the rat[J]. Can J Physiol Pharmacol,2020,98(8):511-521.
nchez-López A,et al. Blocking properties of terguride at the 5-HT2 receptor subtypes mediating cardiovascular responses in the rat[J]. Can J Physiol Pharmacol,2020,98(8):511-521.
[44] Janssen W,Schymura Y,Novoyatleva T,et al. 5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure[J]. Biomed Res Int,2015,2015:438403.
[45] Ayme-Dietrich E,Lawson R,C té F,et al. The role of 5-HT receptors in mitral valvulopathy:bone marrow mobilization of endothelial progenitors[J]. Br J Pharmacol,2017,174(22):4123-4139.
té F,et al. The role of 5-HT receptors in mitral valvulopathy:bone marrow mobilization of endothelial progenitors[J]. Br J Pharmacol,2017,174(22):4123-4139.
[46] Cavero I,Guillon JM. Safety pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy[J]. J Pharmacol Toxicol Methods,2014,69(2):150-161.
[47] Ram P,Penalver JL,Lo KBU,et al. Carcinoid heart disease:review of current knowledge[J]. Tex Heart Inst J,2019,46(1):21-27.
[48] Drake WM,Stiles CE,Bevan JS,et al. A follow-up study of the prevalence of valvular heart abnormalities in hyperprolactinemic patients treated with cabergoline[J]. J Clin Endocrinol Metab,2016,101(11):4189-4194.
[49] Ayme-Dietrich E,Lawson R,Da-Silva S,et al. Serotonin contribution to cardiac valve degeneration:new insights for novel therapies?[J]. Pharmacol Res,2019,140:33-42.
[50] Marzak H,Ayme-Dietrich E,Lawson R,et al. Old spontaneously hypertensive rats gather together typical features of human chronic left-ventricular dysfunction with preserved ejection fraction[J]. J Hypertens,2014,32(6):1307-1316.
[51] Brattelid T,Qvigstad E,Birkeland JA,et al. Serotonin responsiveness through 5-HT2A and 5-HT4 receptors is differentially regulated in hypertrophic and failing rat cardiac ventricle[J]. J Mol Cell Cardiol,2007,43(6):767-779.
[52] Birkeland JA,Swift F,Tovsrud N,et al. Serotonin increases L-type Ca2+ current and SR Ca2+ content through 5-HT4 receptors in failing rat ventricular cardiomyocytes[J]. Am J Physiol Heart Circ Physiol,2007,293(4):H2367-H2376.
[53] 刘敏科,金华.心血管疾病与肠道菌群和5-羟色胺信号系统关系的研究现状[J].中国临床药理学杂志,2020,36(23):3943-3946.
[54] Amsallem E,Kasparian C,Haddour G,et al. Phosphodiesterase Ⅲ inhibitors for heart failure[J]. Cochrane Database syst Rev,2005,25(1):CD002230.
[55] Nebigil CG,Maroteaux L. Functional consequence of serotonin/5-HT2B receptor signaling in heart:role of mitochondria in transition between hypertrophy and heart failure?[J]. Circulation,2003,108(7):902-908.
[56] Saini HK,Sharma SK,Zahradka P,et al. Attenuation of the serotonin-induced increase in intracellular calcium in rat aortic smooth muscle cells by sarpogrelate[J]. Can J Physiol Pharmacol,2003,81(11):1056-1063.
[57] Brasil D,Temsah RM,Kumar K,et al. Blockade of 5-HT(2A) receptors by sarpogrelate protects the heart against myocardial infarction in rats[J]. J Cardiovasc Pharmacol Ther,2002,7(1):53-59.
[58] Kjekshus JK,Torp-Pedersen C,Gullestad L,et al. Effect of piboserod,a 5-HT4 serotonin receptor antagonist,on left ventricular function in patients with symptomatic heart failure[J]. Eur J Heart Fail,2009,11(8):771-778.